The coffee-ring effect
Have you ever observed how a drop of coffee dries? As water evaporates, its suspended particles are deposited in a ring-like fashion in a phenomenon known as the coffee-ring effect. Obviously, this effect is undesirable in the numerous practical applications that require a uniform coating. However, the way to avoid it has remained unknown. The experimental work by 1 proves that the use of ellipsoidal particles –instead of or in addition to spherical particles- eliminates the coffee-ring effect, ensuring a uniform deposition during drop evaporation.
Experiments
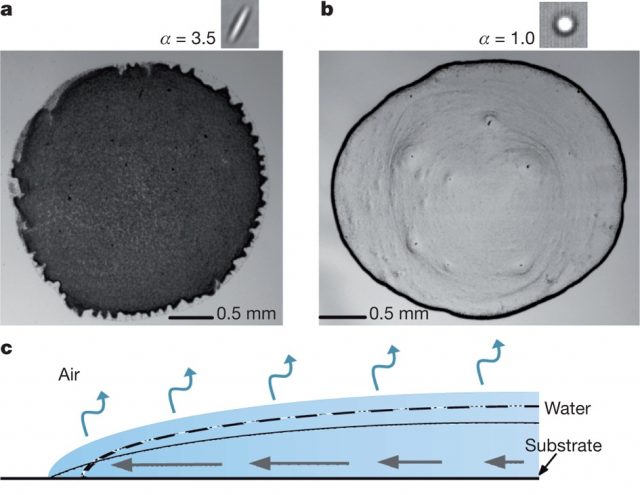
The experiments were conducted with water droplets containing a suspension of polystyrene particles with a size in the range of micrometres, and different major- /minor-axis aspect ratios (α): spheres (α=1.0), slightly deformed spheres (α=1.05, 1.1, 1.2, 1.5) and ellipsoids (α=2.5, 3.5). The particle volume fraction was varied from 10-4 to 0.2. Drops were evaporated on glass slides and the whole process was recorded using video microscopy.
The drying process
Evaporation occurs over the entire drop surface (see blue arrows in Fig. 1c). If the contact line were free to recede, the drop would shrink while keeping its profile (dashed line), which is determined by the surface tension. However, since the contact line remains pinned to the surface, the contact angle is forced to decrease (solid line), inducing a capillary flow (black arrows) from the drop’s centre to the edge to replenish the fluid evaporated at the contact line.
The contact line pinning was studied using video microscopy to measure drop radius during evaporation of different suspensions, and it was concluded that in all cases the contact line remains pinned until the final stage of evaporation.
Evaporation rates were also determined by measuring drop mass of different suspensions during the evaporation process. The drop mass was found to decrease linearly with time. The evaporation rate was the same for drops of sphere or ellipsoid suspension, and drops of pure water.
In short, experimental measurements proved that the drying of droplets with either spherical or ellipsoidal particles exhibit similar contact line behaviour, capillary flow, and evaporation rates. Nevertheless, the deposition of both suspensions is entirely different: spheres are carried to the drop’s edge forming a ring after evaporation is complete (Fig. 1, a), while ellipsoids pack on the interface and produce a uniform deposition (Fig. 1, b).
The reason for this packing is that, once the ellipsoids are transported to the interface, they experience strong attractions to other ellipsoids, more than two orders of magnitude stronger than the attraction between spheres. Then, the energy cost of deforming, moving or breaking up these clusters of ellipsoidal particles is large enough to resist the shear from the radially outward flow. The lower attractive force between spheres is not strong enough to prevent the radially outward fluid flow pushing them to the drop’s edge.
To confirm that deformations of the interface are responsible for the uniform deposition of ellipsoids, a small amount of surfactant was added to a suspension of ellipsoids. The decrease of surface tension produced by the surfactant made interfacial deformations less energetically expensive, restoring the coffee-ring effect. In this case, ellipsoids did not deform the air–water interface and their mutual interactions were also reduced; so they packed similarly to spheres.
What about a mixture of ellipsoids and spheres?
Lastly, Yunker et al. prove that the addition of small numbers of ellipsoids to sphere suspensions can also suppress the coffee-ring effect.
On the one hand, if the diameter of the spheres is smaller than the minor axis of the ellipsoids, then the coffee ring still occurs. The main reason is that the spheres are able to move under or through the particle structures and reach the drop’s edge (Fig. 2 h–j).
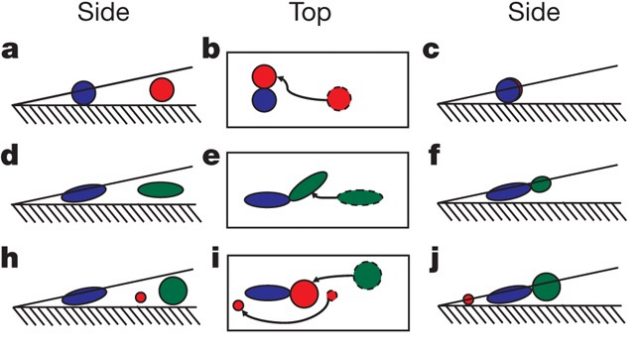
On the other hand, if the spheres’ diameter is larger than the minor axis of the ellipsoids, the coffee ring effect diminishes as the ellipsoid volume fraction is increased, until it eventually disappears. In this case, the spheres join the loosely packed particle aggregates, eliminating the coffee-ring effect (Fig. 2 h–j).
Conclusions
Yunker et al. have proved experimentally that ellipsoidal particles sit at the air–water interface, whereas spheres do not and are carried all the way to the contact line.
They also showed that the addition of small numbers of ellipsoids to sphere suspensions also suppresses the coffee-ring effect, as long as the diameter of the spheres is larger than the minor axis of the ellipsoids.
Besides the fact that ellipsoidal particles avoid the ring effect, this work suggests that other methods based on inducing strong capillary interactions, for example, surface roughness, could also be used to ensure uniform coatings.
References
- P. J. Yunker, T. Still, M. A. Lohr, A. G. Yodh, (2011). Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 476, 308. ↩
1 comment
[…] https://mappingignorance.org/2013/06/07/the-coffee-ring-effect/ […]