Carbon nanotubes to study neuron activity

Human brain has about 85 billion neurons. Each neuron forms thousands of chemical and electrical synapses with other neurons. To record the synaptic activity of each neuron in the brain an intracellular probe with a millivolt scale is required. Glass electrodes are widely used, but they are fragile and they have high impedance. An intracellular electrode made out of pure carbon nanotubes (CNTs) have improved electrical and mechanical properties, but requires a relatively long insulated shaft to penetrate into brain tissue and an exposed tip of sub-micron diameter to impale and stably record from neuronal cell bodies. Researchers from Duke University (Durham, North Carolina, USA) report in PLoS ONE 1 a simple, millimeter-long electrode with a sub-micron tip, fabricated from self-entangled pure CNTs to record neuron activity in vitro and in vivo. Inho Yoon and his colleagues have developed a CNT probe acting like a needle, first sinking into the neurons and then collecting the electrical signals they send to communicate with other neurons.
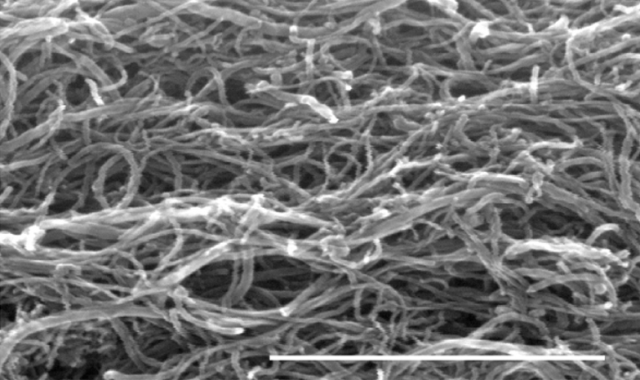
Carbon nanotubes are cylinders of one or more layers of graphene (a hexagonal lattice of carbon atoms with only a single atom of thickness). Single-walled CNTs have a diameter between 0.8 and 2 nm, and multi-walled CNTs (MWCNTs) larger than 5 nm, exceeding even 100 nm. CNT lengths range from less than 100 nm to several centimeters. Researchers can manipulate nanotubes one-by-one using an atomic force microscope, but the process is time-consuming and expensive. For commercial applications unorganized CNTs are preferred 2. For this reason, Duke’s team has fabricated CNT probe by using the technique known as dielectrophoresis, which results in a self-entangled MWCNT mess illustrated in Figure 2.
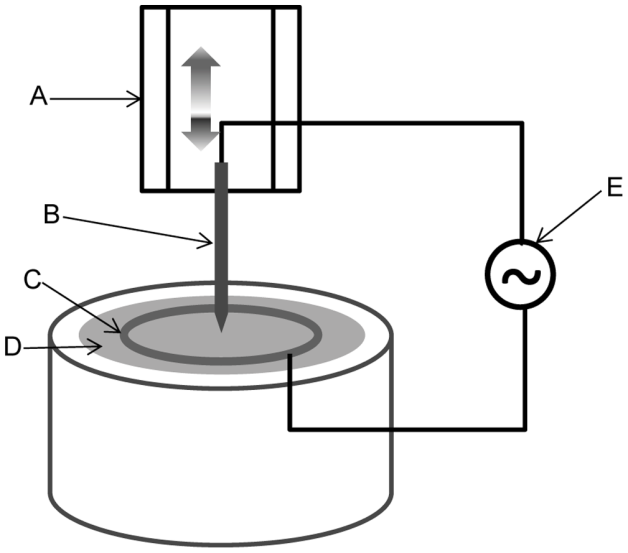
In the dielectrophoresis process, an electrochemically sharpened tungsten wire (diameter 125 µm) is introduced into a solution with MWCNTs of outer diameter 8–15 nm. The sharp tip of the tungsten wire was placed to touch the solution in the middle of a metal ring acting as counter electrode (see Figure 3). A sinusoidal 10 MHz signal, 40–80 V peak to peak amplitude, is applied between the tungsten wire and the counter electrode, while the wire was slowly pulled (40 µm/sec) out of the solution. The result is the grown of a pure MWCNT shaft of about 1 mm length and 5–10 µm diameter in the tip of the wire. By increasing the pulling speed during the last 100 µm the tapering of the shaft down to a single CNT is achieved. To stabilize the electrochemical performance and mechanical stiffness of the CNT probe a 300 nm thick Parylene-C coating was applied along the length of the probe’s shaft.
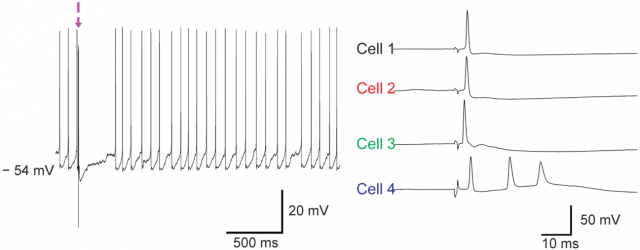
The CNT probe can be used for both intracellular and extracellular recording of neuron activity. Inho Yoon and his colleagues performed intracellular recordings in vitro using neurons extracted from brain slices of transgenic mice whose neurons have a light-activated ion channel. The blue light stimulation of these neurons depolarizes the cells in vitro and lead to firing of action potentials. Figure 4 shows intracellular recordings from a total of four neurons using three different CNT probes (out of 6 CNT probes), with a mean recording time of 3 minutes. Successful penetration was accompanied by a sharp drop in membrane potential, and enabled the detection of spontaneous synaptic potentials and action potentials.
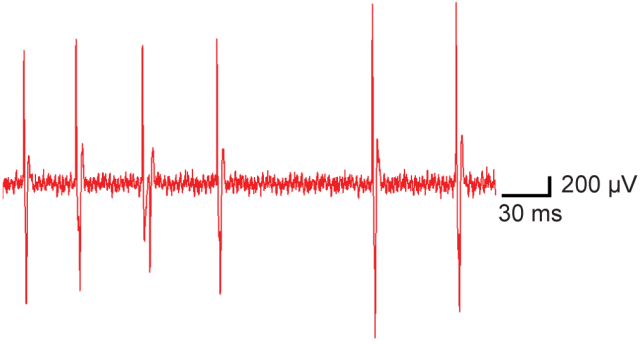
Duke’s team have also successfully made extracellular recordings of neural activity in the somatosensory cortex of anesthetized mice. By using seven CNT probe samples extremely large amplitude single unit events with large signal to noise ratio have been observed, as illustrated in Figure 4. Although intracellular recording configurations were not achieved, the successful intracellular recordings made in brain slices suggest that such recordings are feasible using CNT probes in vivo.
In summary, the novel carbon nanotube probe suitable for intracellular and extracellular recordings from vertebrate neurons both in vivo and in vitro is a good proof of principle. Lower impedance than glass sharp recording electrodes, mechanical flexibility, and bio-compatibility are their main advantages. Moreover, the fabrication technique can been easily extended to the fabrication of microelectrode arrays containing a large number of CNT shanks through which neural signals can be recorded simultaneously in a large number of neurons. Furthermore, advances in this technology could be quite helpful not only for mapping the brain, but also for developing (invasive) brain-computer interfaces.
References
- Inho Yoon, Kosuke Hamaguchi, Ivan V. Borzenets, Gleb Finkelstein, Richard Mooney, Bruce R. Donald, “Intracellular Neural Recording with Pure Carbon Nanotube Probes,” PLoS ONE 8(6): e65715, June 19, 2013. DOI: 10.1371/journal.pone.0065715. ↩
- Michael F. L. De Volder, Sameh H. Tawfick, Ray H. Baughman, A. John Hart, “Carbon Nanotubes: Present and Future Commercial Applications,” Science 339: 535-539, 1 Feb 2013. DOI: 10.1126/science.1222453 [free PDF]. ↩