Sieving at the nanoscale: desalination of seawater through nanoporous graphene
Perhaps the most repeated words in the last few years when talking about graphene – since scientists Geim and Novoselov were awarded the Nobel Prize in Physics in 2010 for their groundbreaking experiments – are “the material of the future”. There are some risks regarding so many expectations about everything related to materials science, since similar breakthroughs have ended up confined to the limits of the lab bench. Nevertheless, the outstanding properties of this one-atom thick 2D lattice of carbon atoms promise equally outstanding developments in the future.
In the meantime, new and exciting potential uses appear from time to time that keep us in suspense. One of the prospective uses currently attracting more attention is that of the nanoporous graphene materials. An interesting review1 recently published in Materials Today examines the last discoveries both in the production and application of this graphene-based nanocellular structure. Nanoporous graphene consists of a two-dimensional graphene sheet in which a pattern of nano-sized porous distribution has been achieved by means of different techniques, among them, electron-beam irradiation or chemical vapor deposition.
Creating a porous structure in any kind of material always leads to new interesting properties. We have learned a lot from nature, as porous structures are constantly appearing in naturally occurring materials such as bones or wood. In the last few years materials scientists are paying much more attention to the properties arising from materials with nano-sized porosity, also known as nanofoams. In the case of graphene, this nanoporosity seems to considerably widen its range of uses. To name a few, it opens the band gap of the graphene sheets, allowing its use in field effect transistors (FETs), very limited in pristine graphene due to its zero band gap; moreover, the presence of porous in its structure increases the surface area per volume which in turn increases the content of edges acting as adsorbing sites suitable for molecular sensing applications.
But without any doubt, one of the most remarkable uses of nanoporous graphene might well be that of selective molecular sieving. There are several properties that make nanoporous graphene the ultimate selective membrane: its exceptional mechanical strength, its atomic thickness and the possibility to physically or chemically modify the graphene pores in order to create different barriers for different molecules.
As soon as this potential use of nanoporous graphene as a filter for certain molecules was envisaged, the possibility of applying this new material to desalination of seawater became a focal point for materials scientists. It is well known that the shortage of water resources for human activities is one of the most urgent problems worldwide, while oceans and seas contain around 97% of the planet’s water. That’s why desalination could become an ultimate solution where depletion and deterioration of water resources are already unavoidable.
However, as easy as this solution could appear at first sight, desalination technologies still require high technical investments and large energy consumption. The most efficient and cost-effective technology at the moment is that of reverse osmosis (RO), but the transport of water across membranes using RO is still quite slow. It seems that the use of nanoporous membranes as filters would considerably speed up this water flow through the nano-sized channels. Furthermore, taking into account that the flux across a membrane scales inversely with the membrane’s thickness, the one-atom thick graphene structure would become the ideal material for this purpose.
Like most of the ongoing studies related to nanoporous graphene, its use for desalination of sea water has been analyzed computationally, mostly using classical molecular dynamics simulations. That is the case of a very well known study from Professor Jeffrey C. Grossman2, from the Massachusetts Institute of Technology (MIT), in which a complete study of the nanoporous graphene behaviour has been carried out mainly considering three critical parameters: pore size, chemical functionalization of pore’s edges and applied pressure over the membrane.

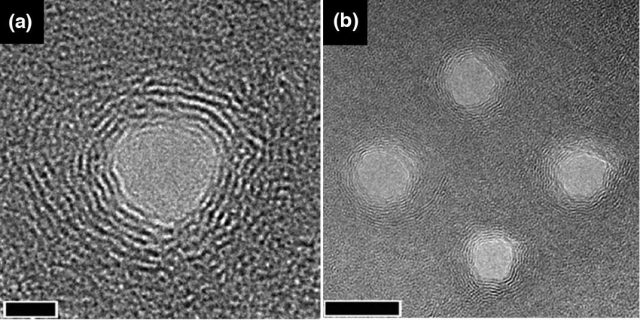
Chemical functionalization of pore’s edges has been proved to have an important impact in the flux across nanoporous membranes. In this study, they altered the pore chemistry using both hydrogen groups (H-) and hydroxyl groups (OH-). The hydrogenated pores were obtained by passivating the graphene’s carbon atoms at the pore edge with hydrogen atoms, while hydroxylated pores were obtained by bonding hydrogen groups and hydroxyl groups alternatively to the unsaturated carbon atoms along the pore edge. The result can be seen in figure 4 (a and b).
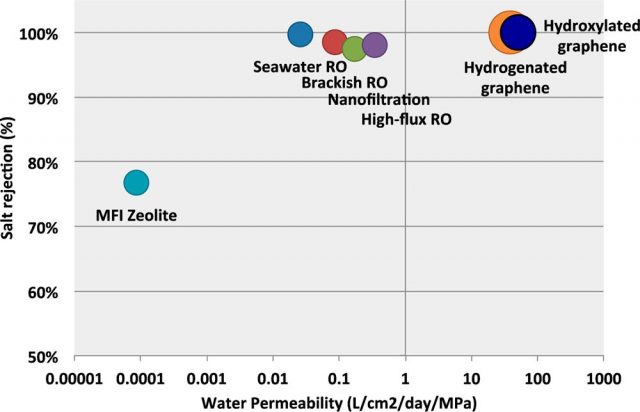
Of course, pore size has to be large enough to allow water molecules flow, but narrow enough to hinder the passage of salt ions. As the pore size increases, the water permeability is higher, and then the water flow speeds up. However, larger pore areas also lead to less effective salt rejection, so that a compromise between water permeability and salt rejection must be achieved.
It seems that hydroxylated pores behave better than hydrogenated pores regarding water permeability. The authors attribute this result to an entropic effect. The hydrophobic nature of H-pores restricts the number of configurations in which water molecules can cross the membrane, while the hydrophilic nature of OH-pores allows different conformations for water molecules inside the pore, then accelerating the water flow. On the contrary, regarding salt rejection, the authors found that H-pores behave better than OH-pores. The latter are more likely to bond with salt ions and, as a result, the free energy barrier to ionic passage is reduced.

All in all, using these chemically modified nanoporous graphene membranes results in an increase of several orders of magnitude in the water permeability than that of the reverse osmosis (RO) membranes. Nevertheless, there are also some critical aspects that will have to be properly improved: first of all, mechanical stability under applied pressure, although inherent in this material, could be improved by adding a support layer to the graphene membrane; on the other hand, a narrower pore size distribution would considerably improve the salt rejection performance of the membrane, allowing lower applied pressures and energy requirements. The authors suggest that the use of improved bottom-up methods in the production of nanoporous graphene will result in a remarkable progress of this kind of structures.
While everybody is waiting for the graphene revolution to translate into real-world applications, the experts claim that graphene market should start to take off after 2015, and it will take some years for all these new technologies to live up to its full potential. Graphene will have to attract technological markets enough for them to make large investments in its mass production and finally allow these high expectations turn into large-scale industrial applications.
References
- Yuan W. & Gaoquan Shi (2014). Nanoporous graphene materials, Materials Today, 17 (2) 77-85. DOI: http://dx.doi.org/10.1016/j.mattod.2014.01.021 ↩
- Cohen-Tanugi D., Grossman J. C., (2012) Water Desalination across Nanoporous Graphene, Nano Letters, 12, p. 3602-3608. dx.doi.org/10.1021/nl3012853 ↩
4 comments
[…] Continue reading . . . […]
[…] is the case of a very well known study from Professor Jeffrey C. Grossman2, from the Massachusetts Institute of Technology (MIT), in which a complete study of the nanoporous […]
Thank you very much. It is very interesting article ..
[…] Sieving at the nanoscale: desalination of seawater through nanoporous graphene […]