Boron and other triel elements as strong Lewis acid centers
Boron, in the periodical table situated in the 13 group (group III according to the former nomenclature), is a semiconductive element with properties between that of a metal and a nonmetal. Boron compounds are widely used materials in various branches of industry. Recent studies show its importance in biology as for example to various metabolic, nutritional, hormonal, and physiological processes.
One can mention the importance of the other 13 group elements (triel elements), aluminium, gallium, indium and thallium. For example, aluminium is the second-most used metal after steel, largely because it is so versatile while nearly all gallium is used today in electronics. Numerous advantageous properties of triel elements are a consequence of the acidity of their compounds – the triel centres are strong Lewis acids in different chemical species. Such acidity of triel centres in simple moieties is briefly described here.
The acidity and basicity are fundamental concepts in chemistry; in terms of the Lewis theory, they are defined as the capacity to accept an electron-pair or to donate an electron-pair, respectively. These terms are very useful to describe inter- and intramolecular interactions. For example, the A-H…B hydrogen bond is the interaction between the B Lewis base centre often characterized by the lone electron pair (the electron-pair donor) and the positively charged H-atom which is the electron-pair acceptor (Lewis acid centre). In a case of the A-X…B halogen bond (X designates the halogen atom) B, similarly as for the hydrogen bond, is the Lewis base centre possessing at least one electron-pair and the X-halogen atom is characterized by the positive electrostatic potential in the elongation of the A-X bond thus it may act as the Lewis acid centre. The A-H…B hydrogen and A-X…B halogen bonds were described in the article ¨The halogen bond – the interaction similar to the hydrogen bond¨ in the Mapping Ignorance website. The Lewis acid – Lewis base interactions are often utilized to interpret the mechanisms of chemical reactions. In case of the borane-based Lewis acids their key role as catalysts in organic synthesis is often analyzed. The strong Lewis acid properties of boron and other triel centres are the result of their electron structure. For example, in trihydridoboron, BH3, and boron trihalides, boron is electron deficient since it has six electrons, i.e. three B-H -bonds, in the outer shell. The similar situation is observed for the analogues triel compounds. The mentioned here electron deficiency concerns the vacancy of the p-orbital being perpendicular to the plane of the molecule (Scheme 1).
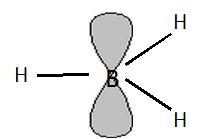
This situation (Scheme1) leads to strong electron accepting properties of triel centres in the direction perpendicular to the molecule considered. This also suggests that the interactions between the triel atoms and Lewis bases are characterized by the dominance of contributions reflecting the electron charge shifts being the result of complexation. Complexation should be understood as a formation of a complex between the Lewis acid unit (here the triel species) and the Lewis base.


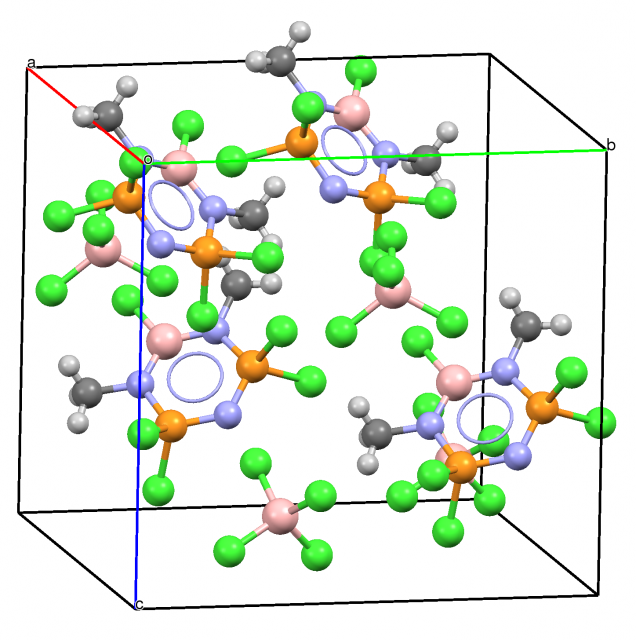
It seems that the electrostatic term is also important for the triel centre – Lewis base interactions. Figure 1 presents maps of the electrostatic potential for the BH3 molecular surface. One can see the positive electrostatic potential region in the direction perpendicular to the molecule what shows that the electrostatic interactions are possible here with the species characterized by the negative electrostatic potential (usually Lewis base centres). However the vacant p-orbital in triel compounds (Scheme 1) is very ¨eager¨ to be filled thus the large electron charge density shifts from the Lewis base to the triel species often lead to the increase of the valency from three to four. Such a situation occurs if trivalent triel centre interacts with strong Lewis base. The interaction of the BCl3 molecule with Cl– anion is an example since it leads to the formation of the BCl4– ion. Such types of interactions are often observed in crystal structures, Figure 2 shows an example of the crystal structure containing the BCl4– ion.
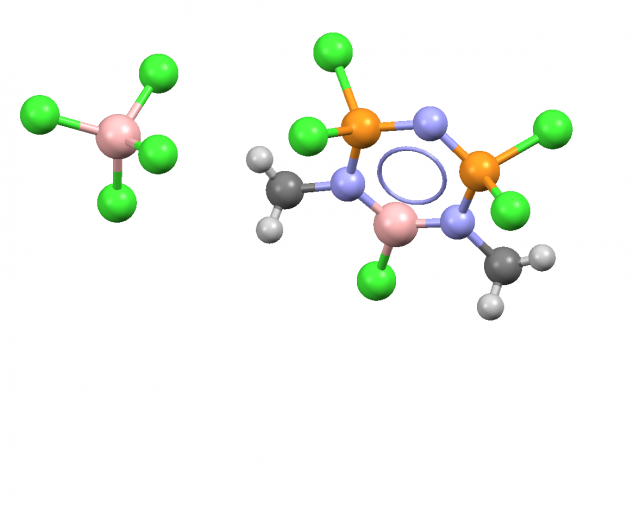
This is interesting that usually the interactions of trivalent triel centres with Lewis bases lead to the creation of a next covalent bond, i.e. to the increase of the valency of the triel atom or at least for the trivalent triel atom the additional Lewis acid – Lewis base link possesses the properties of the covalent bond is formed. Figure 3 presents the crystal structure reflecting such a situation where the (BCl3)(C2H5)2O species may be considered as the product of the reaction of the trichloro borane, BCl3, with the diethyl ether, (C2H5)2O.
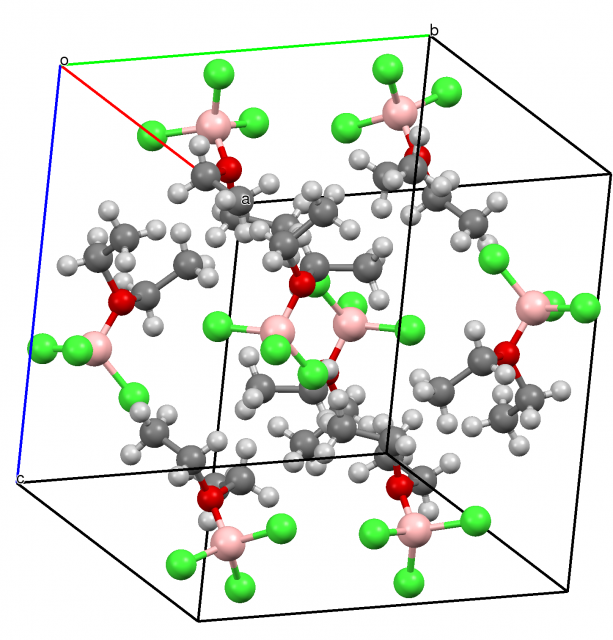

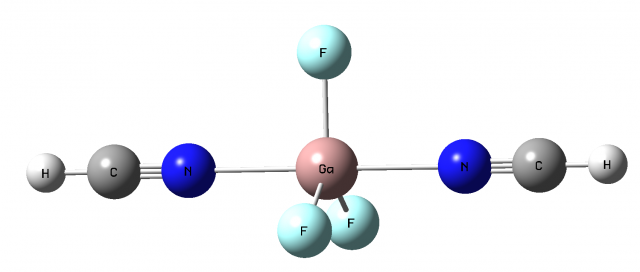
Sometimes the increase of the valency of the triel atom up to five may be observed. For example, pentacoordinate boron compounds have been postulated early as transition states of SN2 reactions. The reaction of the BH3-CO species with the N(CH3)2 is an example of such a situation 12. Note that there is formally vacant p-orbital for the BH3 molecule, for the boron trihalides and for other triel analogues, i.e. two p-orbital lobes are observed (see Scheme 1). This means that the nucleophilic attack, i.e. the interaction with the Lewis base, may be observed in two directions since the Lewis base may approach each of lobes of the vacant p-orbital. Such a situation was analyzed recently theoretically at the MP2/aug-cc-pVTZ level of approximation 3. Figure 4 presents the GaF3(NCH)2 species being the result of interaction of GaF3 with HCN in two directions mentioned earlier here. One can see that the GaF3(NCH)2 moiety is characterized by the planar GaF3 central part and two HCN ligands forming links with the Ga-centre. Hence the whole structure may be classified as the trigonal bipyramid. Such structures are typical for the transition state of the SN2 reaction 4 but the GaF3(NCH)2 complex corresponds to the energetic minimum. On the other hand the mentioned above complex of the BH3-CO species with the N(CH3)2 molecule analyzed experimentally reflects the situation of the transition state. However the mechanism of the electron charge shift is the same for both cases. There is the electron charge shift to one lobe of the p-orbital of boron from the CO ligand and to the second lobe of boron from the electron-pair of the N(CH3)2 molecule for the transition state structure.
It is important for the formation of the pentavalent triel centre that the ligands interacting with the triel atom should be relatively strong Lewis bases but not very strong. For example for the BF3 molecule interacting with very strong F– Lewis base the BF4– anion is the product of reaction. The latter species can not interact further with the next Lewis base ligand to form the pentavalent boron species since the whole BF4– molecular surface is characterized by the negative electrostatic potential. Additionally, because of the steric effects there is no an access to the tetrahedral B-centre for the next ligand. In the case of weaker HCN or N2 Lewis bases the pentacoordinated triel atom may be formed (Fig. 4).
Hence the existence of pentavalent triel atom is rather rare but such cases were described in literature. Recently four types of ligand motifs for pentacoordinate boron systems were presented 5 or crystal structures of the pentacoordinate and tetracoordinate boron and carbon compounds were analyzed 67.
This is worth to mention that for the trihydridotriel species and the triel trihalides mentioned here as well as for other numerous triel compounds the phenomenon of hypovalency occurs since the trigonal triel atom is characterized by six electrons in the outer shell; it means that the trigonal triel atom does not obey the octet rule. For the tetrahedral triel atoms this rule is obeyed (eight electrons in the outer shell) while for the pentavalent triels (the trigonal bipyramid structure) with ten electrons in outer shell the phenomenon of hypervalency occurs and again the octet rule is not obeyed.
References
- ¨Reactions of Co-ordinated Boron Compounds in the Gas Phase. Part I. Borine Carbonyl and Trimethylamine¨, J. Grotewold, E. A. Lissi, A. E. Villa, J. Chem. Soc. A1966, 1034-1037. ↩
- ¨Reactions of Co-ordinated Boron Compounds in the Gas Phase. Part II. Triethylarnine as Scavanger of Borine¨ J.Grotewold, E.A.Lissi, A.E.Villa, J. Chem. Soc. A1966, 1038-1041. ↩
- Grabowski S.J. (2014). Boron and other Triel Lewis Acid Centers: From Hypovalency to Hypervalency, ChemPhysChem, 15 (14) 2985-2993. DOI: http://dx.doi.org/10.1002/cphc.201402344 ↩
- ¨Advanced Organic Chemistry¨ J.March, , 4th ed., Wiley, New York, 1992. ↩
- ¨Synthesis and Characterization of Terpyridine-Supported Boron Cations: Evidence for Pentacoordination at Boron¨, G.P.McGovern, D.Zhu, A J.A.Aquino, D. Vidović, M.Findlater, Inorg. Chem. 2013, 52, 13865-13868. ↩
- ¨Syntheses and Structures of Hypervalent Pentacoordinate Carbon and Boron Compounds Bearing an Anthracene Skeleton – Elucidation of Hypervalent Interaction Based on X-ray Analysis and DFT Calculation¨, M.Yamashita, Y.Yamamoto, K.Akiba, D.Hashizume, F.Iwasaki, N.Takagi, S.Nagase, J. Am. Chem. Soc. 2005, 127, 4354-4371. ↩
- ¨Synthesis and Characterization of Stable Hypervalent Carbon Compounds (10-C-5) Bearing a 2,6-Bis(p-substituted phenyloxymethyl)benzene Ligand¨, K.Akiba, Y.Moriyama, M.Mizozoe, H.Inohara, T.Nishii, Y.Yamamoto, M.Minoura, D.Hashizume, F.Iwasaki, N.Takagi, K.Ishimura, S.Nagase, J. Am. Chem. Soc. 2005, 127, 5893-5901. ↩
2 comments
[…] eta konposatuen egonkortasunari buruz ari bagara. Slawomir Grabowskik erakusten digu Boron and other triel elements as strong Lewis acid centers artikuluan, erregelak, erregelak direla eta, hauek, ez direla legeak salbuespenak […]
[…] reactividad de los elementos y la estabilidad de los compuestos. Slawomir Grabowski nos enseña en Boron and other triel elements as strong Lewis acid centers que las reglas se llaman reglas y no leyes porque tienen […]