Polyoxometalates, the other discovery by D’Elhuyar brothers
Not many people know that polyoxometalates (POMs) discovery dates back to 1783 and that they were the D´Elhuyar brothers (Juan José D´Elhuyar(Logroño, 1754) and Fausto D´Elhuyar (Logroño, 1755) who discovered a yellow spicy/bitter tasting salt from the reaction of ammonium molybdate with phosphoric acid which is now known as ammonium 12-phosphomolybdate, (NH4)3[PMo12O40] 1 (Figure 1).

Although in 1826 it was Berzelius who first reported the system 2. And In 1933, the structure H3[PW12O40]was solved by Keggin by powder X-ray diffraction, reporting the first polyoxoanion which is now known as Keggin polyanion 3.
What are they?
They are discrete structurally and chemically diverse nanosized metal-oxygen molecular anions of early transition metals (groups V and VI) in their highest oxidation states 4.They have a wide range of molecular structural diversity, existing in a wide variety of shapes, sizes and compositions. They are composed by the condensation of MOn units, where ‘n’ indicates the coordination number of M (n = 4, 5, 6 or 7).
How can we describe them?
In general, POMs, can be described by the formula [XxMmOy]q– where X is the heteroatom and M is a transition metal of V and VI groups, principally Mo, W and V and less frequently Nb, Ta, Cr. There are no limitations for the heteroatom as about 70 elements of the periodic table are known, with the exception of noble gases.
How can we classify them?
Isopolyoxometalates, [MmOy]n−, are metal oxide cluster species that are composed of a single metal type, with no additional heterogroups or supporting ligands.
Heteropolyoxometalates, are represented with the general formula [XxMmOy]q–where the heteroatom X, can be one of the many elements of the periodic table. It is the most explored group inside POM chemistry, with more than 5000 articles in the past four years.
Molybdenum blue and brown reduced POMs, inside this group, ring or sphere shape giant clusters are found, composed by a combination of Mo(VI) and reduced Mo(V) centers and connected by oxygens. Mo-Blues are the most remarkable species of polyoxoanion generated by self assembly procedures by the condensation of small buildings blocks to giant discrete molecules up to 368 molybdenum centers described by Prof A.Muller 5 (Figure 2).

Which is the most studied structure?
The Keggin structure is the most widely studied structure for tungsten POMs. It has the general formula [XM12O40]n– (heteropolyanion) where most commonly metals (M) are MoVI or WVI and the heteroatom (X) are BIII, AlIII, SiIV, PV, FeIII, GeIV and AsV. It is based on a central heteroatom XO4 tetrahedron surrounded by four trimers, M3O13 groups, of three edge-shared MO6 octahedra. These trimers are linked via corner-sharing to each other and to the central XO4 tetrahedron via μ2– and μ4– oxo bridges respectively, resulting in a high symmetry (Td) as shown in Figure 3.
![Figure 3. The four M3O13 triads and one XO4 tetrahedron generate the Keggin-type [XM12O40]n− heteropolyanion.](https://mappingignorance.org/app/uploads/2015/04/Diapositiva3-640x357.jpg)
Keggin-type heteropolyanions can have five different rotational isomers known as Baker-Figgis 6. The α isomer has the overall Tdsymmetry. By a 60° rotation of one, two, three or all four trimers, the isomers β, γ, δ and ε can be formed,generating structures of C3v, C2v, C3v and Td symmetry respectively (Figure 4). In general, γ, δ and ε are less stable than α and β isomers because some of the trimers are edge-sharing, decreasing linear M-O-M bonds and so dπ-pπ interactions 7.
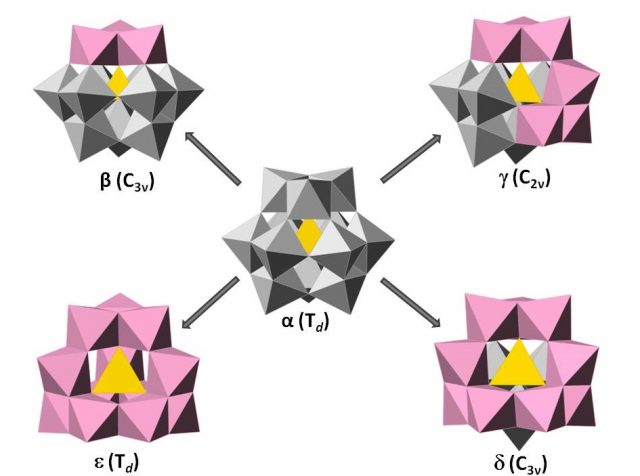
Lacunary species or vacant species of the Keggin structures are obtained by removal of one, two, or three MO6 octahedra under basic conditions forming what so-calledmono-, di-, and tri-lacunary species. There are only known lacunary species of the Baker-Figgis isomers, α, β and γ.
The plenary α–[XM12O40]n– Keggin is the most stable species in acid media but it can lose adjacent MO6 octahedra to form mono- and tri-lacunary species by increasing the pH of the solution. The monovacant {α-XM11O39} is generated when removing one of the twelve equivalent octahedra. The trilacunary species can be either {A-α-XM9O34} in which three corner-shared octahedral (triad, M3O15) have been lost or {B-α-XM9O34} in which three edge-shared octahedral (trimer, M3O13) are removed. In contrast, due to C3v symmetry of the β isomer, the octahedral are non-equivalent so this implies the formation of different types of lacunary species depending on the position and the type of octahedra removed. Thus, three monovacant species can be generated: {β1-XM11O39}, if the vacancy is located in the opposite triad to the rotated one (unconnected with the rotated triad), {β2-XM11O39} when the vacant is located in the central belt (connected with the rotated triad), and {β3-XM11O39} by the removal of one of the three octahedra that is part of the rotated trimer. In the same way, two trilacunary species can be obtained from the plenary β isomer. {A-β-XM9O34} by removal the opposite triad to the rotated trimer and {B-β-XM9O34} any trimer can be removed with the exception of the rotated one.The removal of two adjacent octahedra from α– and β–[XW12O40]n− isomers has not been observed. But, in the case of γ isomer the dilacunary species, {γ-XM10O36}, is only generated when removing two vertex-sharing octahedral belonging to two rotated trimers (Figure 5).

The lacunary species can be formed either by the acidification of solutions containing X and M salts or by the basification of the heteropolianion XW12O40, breaking M-O bonds. The plenary polyanions are predominant in a very acid pH (0-2), the monolacunary species in a lightly acid pH (4-6) and the trilacunary ones at a basic pH (8-10).
Where can they find applications?
Given the vast array of structures we find applications in many different fields like catalysis, biomedicine or material science, to name a few. I am more familiar with this last case, where POMs act as inorganic ligands and with the inclusion of metalorganic units, the generation of new hybrid materials is guaranteed. The development of such compounds, appear as a creative alternative for obtaining new materials with unusual features and properties8.
References
- [De Luyart Lubice, J. J.; De Luyar Lubice, F. C. Extractos de las Juntas Generales celebradas por la Real Sociedad Bascongada. 1783, 46. ↩
- Berzelius, J. J. Pogg. Ann. Phy. 1826, 6, 369. ↩
- (a) Keggin, J. F. Nature 1933, 131, 908. (b) Keggin, J. F. Proc. Roy. Soc. A. 1934, 144, 75. ↩
- Pope, M. T. Springer-Verlag, Berlin, 1983. ↩
- (a) Muller, A.; Beckmann, E.; Bogge, H.; Schmidtmann, M.; Dress, A. Angew. Chem. Int. Ed.2002, 41, 1162. (b) Richmond, C. J.; Miras, H. N.; Ruiz de la Oliva, A.; Zang, H.; Sans, V.; Paramonov, L.; Makatsoris, C.; Inglis, R.; Brechin, E.K.; Long, D. L.; Cronin, L. Nat. Chem.2012, 4, 1037. ↩
- Baker, L. C. W.; Figgis, J. S. J. Am. Chem. Soc. 1970, 92, 3794. ↩
- (a) Kepert, D. L. Academic Press: New York, 1972, 46. (b) Pope, M. T. Inorg. Chem. 1976, 15, 2008. ↩
- Dolbecq, A.; Dumas, E.; Mayer, C. R.; Mialane, P. Chem. Rev.2010, 110. ↩
2 comments
[…] Noizbehinka zientziak dituen nazionalismo-parrastadetan (bestalde, zentzugabeak direnak inongo aurkikuntza ez baita modu isolatuan egiten) bi elementu kimikoen aurkikuntza “espainiarrak” direla esaten da, platinoaren eta tungstenoaren aurkikuntza. Lehenengoa oso zalantzazkoa da eta bigarrena, sendoagoa teknizismo batengatik: jakina zen tungstenoa existitzen […]
[…] En los ramalazos de nacionalismo que hay a veces en la ciencia (absurdos, por otra parte, porque ningún descubrimiento se realiza aisladamente) se suele nombrar como “españoles” el descubrimiento de dos elementos químicos, el platino y el tungsteno. El […]