When plants listen

Everybody has heard about that. They say that plants are able to hear. Who has not ever heard from someone or read somewhere that talking to your plants makes them grow better and to be more splendid than if you treat them like a vegetable? You can believe it or not, but believer or skeptic, you may have asked yourself this question: How could they hear without ears and what for? Nowadays, nobody expects plants to have obvious sound receptors, but after centuries studying the plant kingdom still no apparent sound sensor has been found. Furthermore an evolutionary question remains. Sound perception seems to be pretty useful for those living beings able to move in order to hunt or to scape, being always on alert, in fast interaction with the environment. However, what could be the evolutionary utility of this capacity in motionless beings, whose speed and kind of movement, in the best case, allow them to close on themselves, but not to run out of danger.
With time, science has found some evidences about plants reacting against vibrations and a wide range of acoustic energy frequencies (including music), but it is still intriguing the reason of those behaviors in the context of ecology. In a recent paper1, they have found a practical reason for Arabidopsis thaliana to listen, or at least to make use of meaningful environmental acoustic vibrations. In a really smart experimental setup they have been able to observe that those plants react to the vibrations produced by a caterpillar eating leaves. That reaction comes in the form of an improved production of certain chemical compounds known to affect the growth and fitness of many insects.

In fact they have been able to record the vibrations produced for a feeding caterpillar and the wind on the leaves of A. thaliana using laser vibrotomes; and also the song of a leafhopper on another plant. Then later, using that experimental setup they transmitted those vibrations to the leaves (Figure 1). For the chewing and the leafhopper song a piezoelectric actuator was used as high-frequency vibration transmitter. But due to the very low-frequency of wind, the former method was useless and a modified speaker with a stick worked as actuator.
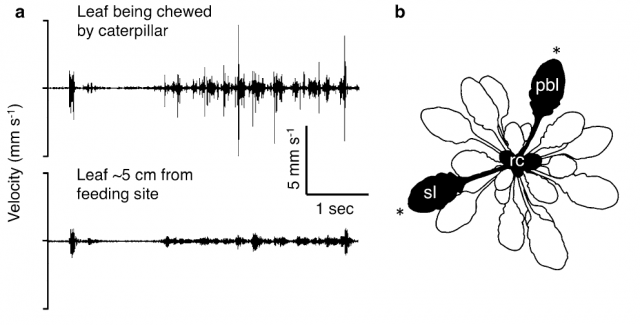
When a caterpillar is chewing a leaf, that vibrational energy is transmitted to other leaves from the same plant (Figure 2). And what Appel and coworkers found is that those leaves exposed to chewing vibrations by playback, and also the systemic leaves from the same plant, produce higher amounts of some chemical defenses like glucosinolates and anthocyanins (Figure 3). But importantly, this effect is observable only when after that “fake exposure” the leaf was chewed by a real caterpillar for a while (allowing it eat up to 30% of the leaf). In the same conditions, vibrations induced by playing back wind or leafhopper songs do not increase the production of those chemical defenses.

However the question remains of whether the observed increase in these chemical defenses is enough to effectively stop the attackers or wether it is ecologically irrelevant. Both glucosinolates and anthocyanin have biological activity oninsects and estimations from the authors based on the observed increase of glucosinolates point to a 20% reduction in growing rate of caterpillars; but it is unclear what would be the biological effect of anthocyanin in this case.
Anyway, it looks like if the sound of a threat would make the plant be more prepared to deal with a new imminent attack. But the mechanism of this priming behavior against herbivores is still unknown. Many hypothesis are still open, it could be that the vibrational information triggers cellular mechanosensors that ultimately elicit the release of phloem-transported chemical signals or volatile hormones. The acoustic energy could also collaborate with other chemical or electric signals released after herbivore-induced damage in order to boost the plant defense system. Furthermore, such a skill could provide to the plant for a fastest and long distance mechanism to transduce signals not only in itself but also through the community of plants via their contacting branches and roots.
Therefore, vibration is suggested as a new player in plant–herbivore insects interaction. Opening up a new field of research in this curious phenomenon that might one day render a pesticide in the form of a compact disc or a music file…
References
- Appel, H. M. & Cocroft, R. B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia175, 1257–66 (2014). ↩
1 comment
[…] Las plantas oyen. Que sí, en serio. Pero, ¿cómo lo hacen si no tienen ni oídos ni cerebro? Daniel Moreno nos lo explica en When plants listen. […]