Contracting layered white graphene with a laser
The exotic properties of layered materials have become a major focus of scientific research. The most famous member of this group is graphene which serves as a building block for few layered graphene and graphite as well as for single- and multi-walled carbon nanotubes. Here, each layer is an one-atom thick hexagonal sheet of sp2 bonded carbon atoms, where the unpaired pz electrons on each atomic site join to form a collective π system turning the material into a semi-metal. Different complex factors interplay dictates the equilibrium interlayer distance and the optimal way of staking, where consecutive layers are shifted with respect to each other such that half of the carbon atoms of one layer reside above the hexagon centres of the adjacent layers.
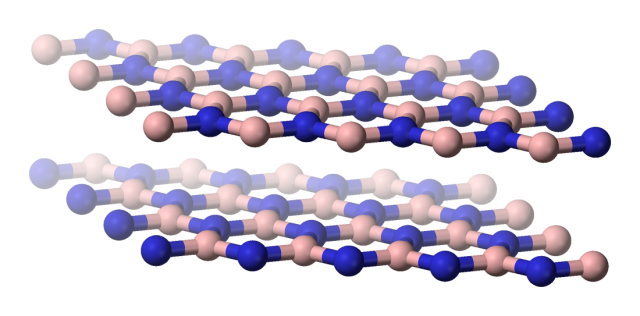
The inorganic analog of graphene, sometimes referred to as white graphene, is hexagonal boron nitride (h-BN). Structurally, a single layer of h-BN is very similar to a graphene sheet having a hexagonal backbone where each couple of bonded carbon atoms is replaced by a boron-nitride pair. Moreover, graphene and h-BN are isoelectronic, but due to the electronegativity differences between the boron and the nitrogen atoms the π electrons tend to localize around the nitrogen atomic centres thus forming an insulating material. Actually, h-BN has recently attracted much attention as a material to be used as insulating junctions for graphene tunnelling transistors and as an insulating substrate for graphene field effect transistors – which show higher performance than those on silicon oxide substrates.
Furthermore, the polarity of the B-N bond results in formal charges around the atomic centres thus allowing for monopolar inter-layer electrostatic interactions to join other interactions in dictating the nature of the interlayer binding. The preferred stacking mode is such that a boron atom bearing a partial positive charge in one layer resides on top of the oppositely charged nitrogen atoms on the adjacent layers.
The nature of the interlayer binding is important because controlling the interlayer distance of layered materials will allow the material properties to be controlled in technological applications.
Now a group of researchers, including Ángel Rubio from Max Planck Institute for the Structure and Dynamics of Matter, Material Physics Center (UPV/EHU-CSIC) and DIPC, has shown1 that an intense infrared laser can be used to compress the interlayer of h-BN, a laser that in normal conditions would cause structural destruction through fast local heating.Contracting the interlayer distance could provide a new route for chemical reactions under pressure.
The team of researchers, instead of using mechanical devices for compression, found using time-dependent density functional theory that laser-stimulated interlayer contraction could produce high-pressure conditions between the atomic layers, thus inducing new phases of intercalated molecules.
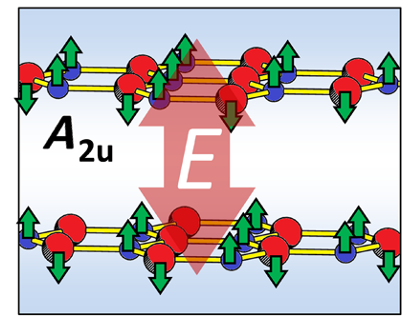
But, which could be the mechanism that would make an usually destructive laser a compressing device? The answer is resonance with phonon excitations. A phonon is the quantum of lattice vibrational energy; it has a magnitude of hf, where h is the Planck constant and f is the frequency of the vibration. When anions (N) and cations (B) in h-BN move in opposite out-of-plane directions, an electric dipole normal to each layer is induced. When the ionic movement is in the same direction in all layers, parallel dipoles are induced in each layer causing interlayer attraction. Such an out-of-plane lattice vibration is made possible with light with an optical frequency f resonant with the vibration. Thus, phonon excitations cause the interlayer contraction.
The interlayer distance of the bilayer h-BN sheet was initially set as 3.274 Å. The researchers found that for the optical frequency resonant a particular lattice vibration, the contraction was large; up to 0.37 Å (11.3% of the original interlayer distance). But the contraction effect is linked to precision: For slightly off-resonance (10%) excitation, the interlayer distance increases toward dissociation of the bilayers.
These are calculations, what could we expect experimentally? Some experiments suggest that it is possible to observe a contraction of 0.17 Å within 1 ps (picosecond) and an estimated retention time of the contraction of 190 fs (femtosecond). The equipment to perform these experiments already exists and the results could be applicable to other bilayered materials, like metal dichalcogenides.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
-
Yoshiyuki Miyamoto, Hong Zhang, Takehide Miyazaki, & Angel Rubio (2015) Modifying the Interlayer Interaction in Layered Materials with an Intense IR Laser Phys. Rev. Lett. 114, 116102 DOI: 10.1103/PhysRevLett.114.116102 ↩