The blue blood of the horseshoe crab
One hears blue blood and immediately thinks about royal family members. Some biologists, though, immediately will think about Limulus polyphemus, a marine animal commonly known as the horseshoe crab. Why? Because, very surprisingly, this arthropod actually has true blue blood! Human blood is red because of the hemoglobin inside our red blood cells, which forms a complex with iron molecules to transport oxygen molecules throughout the body. When the iron is oxygenated, it reflects bright red light and when is deoxygenated, it reflects darker red. However, the horseshoe crab doesn’t use hemoglobin but a different molecule known as hemocyanin. Hemocyanins do not transport oxygen using iron but using copper. Oxygenation of copper causes a color change between the colorless Cu(I) deoxygenated form and the blue Cu(II) oxygenated form1, making the blood of these animals amazingly blue!
This is one of the outlandish features of horseshoe crabs blood that caught the attention of Frederick B. Bang, a pathobiologist who spent his summers doing research on the circulatory system of these arthropods at the Marine Biological Laboratory (MBL) in Woods Hole, MA. One day, Bang found that one of his crabs had died from a strange blood disease in which almost the entire blood volume of the crab clotted into a semi-solid mass. Surprised by that unusual reaction, he dug in further and discovered that somehow it was a reaction to an infection of a type of bacteria known as gram-negative (but never against another type, the gram-positive). Trying to understand the nature of the phenomenon, he injected dead gram-negative bacteria and observed the same effect 2. Since these arthropods don´t possess a complex immune system with antibodies like ours, these discoveries led to the postulation that bacterial-induced blue blood clotting is an ancient immune response developed by these animals to survive against the millions of bacteria found in the sea. And it makes sense. Let’s imagine a horseshoe crab with a sustained a small injury. Seawater comes into contact with the tissue and bacteria come into contact with the blood and begin to infect the body of the crab. That should be stopped as soon as possible and, therefore, rapid clotting of the blood at the injury site avoids further entry of more bacteria.
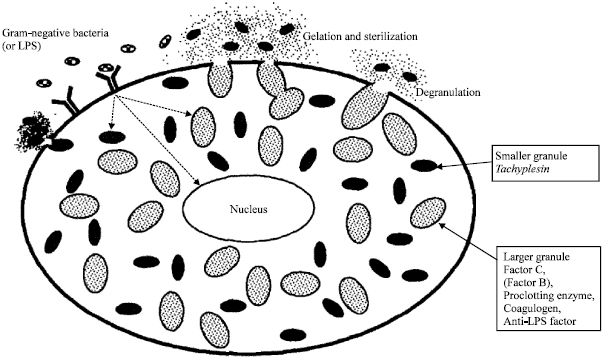
Now we know better how this system works: Limulus blood cells detect a bacterial component, known as endotoxin, which bacteria release continuously into the surrounding medium. Detection of endotoxins by blue blood cells induces the release of the content of internal granules of these cells, which contain coagulogen, a molecule that induces blood clotting3(Figure 1). This simple but successful system has worked for the horseshoe crab for several hundred million years with almost no evolutive changes. One key feature that makes this system successful is its extreme sensibility, being one of the most sensible ways known in nature to detect the presence of bacterial endotoxins. One may not think this is significant but this extreme sensibility to detect bacterial endotoxins is a feature that has impacted tremendously our health and healthcare systems.
Anything that goes into our bodies by injection or during surgery has to be free of bacteria because, if not, the patient will get an infection. But that is not enough. It also has to be free of bacterial endotoxins (even if the product does not have bacteria anymore) because our bodies also respond to endotoxins, generating acute inflammatory responses. Therefore, pharmaceutical companies always needed to test drugs for the presence of endotoxins to be sure that they were not contaminated. Historically this has been done by injecting the drugs in rabbits and observing if fever was developed during the next 48h. This slow method required huge colonies of rabbits, making it really expensive. After the discovery of the clotting properties of the blue blood of horseshoe crabs, a young hematologist named Jack Levin joined Frederick Bang during another summer in Woods Hole to develop an assay to use blue blood to detect endotoxins. The assay consisted of obtaining blood cells (amoebocytes) from Limulus, lysate them (break them) and recollecting the internal components of the cells responsible for clotting. That liquid was named Limulus Amoebocyte Lysate (LAL) and got clotted as soon as endotoxins made contact with it. The use of LAL made really easy to test if other liquids or components contained endotoxins since just adding them to LAL induces its clotting if they contain endotoxins 4 (a series of images describing how LAL is obtained can be found here).

Another scientist at the Woods Hole Oceanographic Institution next door to the MBL, Stanley Watson, realized the potential value of LAL. He founded a company, Associates of Cape Cod, which is now doing a multi-million dollar business extracting blue blood and producing LAL from horseshoe crabs. Nowadays, a quart of horseshoe crab blood has a price tag of around $15,000, leading to overall revenues from LAL industry estimated at USD 50 million per year 5. This is a great example of how basic research (studying the blood of an ancient arthropod) can turn into an unexpected revolution of biomedical science industry. The line between basic research and applied science can really disappear when we try to fully understand unexpected basic discoveries… so promote basic science!
References
- Coates, C. J., & Nairn, J. (2014). Diverse immune functions of hemocyanins.Developmental & Comparative Immunology, 45(1), 43-55. doi:10.1016/j.dci.2014.01.021 ↩
- Bang, F. B. (1956). A bacterial disease of Limulus polyphemus. Bulletin of the Johns Hopkins Hospital, 98(5), 325-351. ↩
- John, A., Khan Chowdhury, A. J., Yunus, K., & Kasim, Z. (2010). Mechanism in the clot formation of horseshoe crab blood during bacterial endotoxin invasion. Journal of Applied Sciences, 10(17), 1930-1936. doi:10.3923/jas.2010.1930.1936 ↩
- Levin, J., & Bang, F. B. (1964). The role of endotoxin in the extracellular coagulation of Limulus blood. Bulletin of the Johns Hopkins Hospital, 115, 265-274. ↩
- Carmichael, R. H., Botton, M. L., Shin, P. K., & Cheung, S. G. (2015).Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management. Springer. ISBN:978-3-319-19542-1 ↩
4 comments
[…] por noesbasura | Feb 22, 2016 | Biology, Noticias, Science | 0 […]
[…] Lo de la sangre azul de los nobles es solo una forma de hablar. Eso sí, hay animales que tienen la sangre azul. Pero azul, azul. Jaime de Juan en The blue blood of the horseshoe crab. […]
[…] un artículo de mappingignorance en […]
[…] The blue blood of the horseshoe crab – Mapping Ignorance […]