Knockout space molecules: much to learn we still have

Author: Giovanna D’Angelo is a Ph.D. student (ITN-EJD-TCCM) at Universitet Stockholm
”Where Do We Come From? What Are We? Where Are We Going?” is the title of a late XIX century famous painting by Paul Gauguin. Even if, to date, Science has not provided a complete answer to all these questions, we are still trying to answer to ”Where Do We Come From?” by figuring out increasingly clearer pictures of the Universe origin and evolution. Well, if we just look up to the sky, those shining lights we can see are telling us stories about far galaxies and billions of years ago.
More still! Our ”space observations” have gone so far that the idea of a chemically empty interstellar space is over today. There are instead evidences for the presence of several molecules, actually rather complex ones as aminoacids or porphyrins (Figure 1, 1). In particular, if we take a look to the infrared spectra from the region between stars, commonly called InterStellar Medium (ISM), we will notice that there is a group of molecules, which goes under the name of Policyclic Aromatic Hydrocarbons (PAHs) (Figure 2a), that is really ubiquitous (Figure 2b).
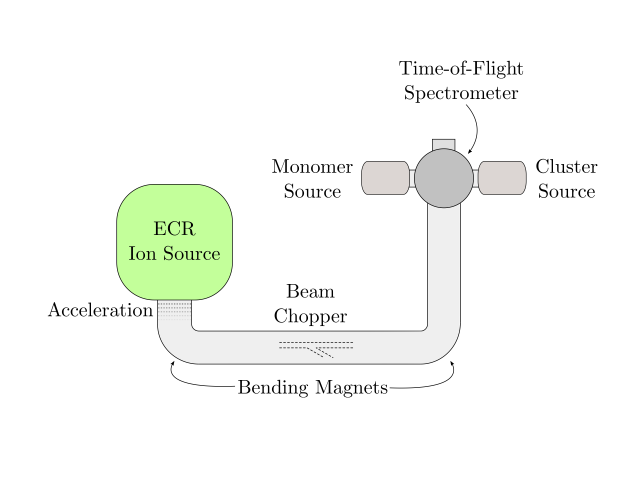
Molecules in the Universe can be the subject of a complex energetic processing. So far, we know that radiation, electrons or atom/ion impact can excite molecules and the ”cooling down” process can occur in different ways. To the best of our knowledge, the most interesting phenomenon for our understanding of the origins of Universe happens to be a very local exchange of energy from an ion to a molecule in a collision at a specific velocity, which can result in the knockout of one or several atoms from the molecule. Chen et al. demonstrated that charged PAHs with a carbon loss can form new covalent bonds easier than neutral entire PAHs 2. In fact both experiments and simulations performed by Gatchell et al. on pyrene clusters colliding with He and Ar ions showed strong evidence for molecular growth, which means that inside a cluster of molecules new bigger molecules are formed. Such experiments were performed at ARIBE beamline at GANIL in Caen, France (Figure 3).
Unfortunately, mass spectra (relative intensity of the signal vs mass/charge of each fragment detected) obtained with these experiments do not give an idea of the structures of the cations detected. In order to inspect the fine chemistry of these processes experiments strongly benefited of a computational modelling. Nowadays it is in fact possible, with the help of computers and dedicate programs, to reproduce entire sequences of a wide range of chemical processes, even though for limited timescale and with variable accuracy. These last factors, in particular, mainly depend on the numerical complexity of the chosen method. In this case the events in the collision chamber were reproduced using Molecular Dynamics (MD), mainly classical dynamics, with semi-empirical potentials, Adaptative Intermolecular Reactive Bond Order (AIREBO) potentials, suitable for reactions of hydrocarbons in gas phase, and Ziegler-Biersack-Littmark (ZBL) commonly used for modelling collisions. 100000 single collision events have been simulated to collect a statistic, featuring different trajectories of the projectile, which are randomly selected. Figure 4 shows that there is a good agreement between experimental (on the left) and computational (on the right) results for collisions with Ar (top row) and He (bottom row) projectiles at selected energies.
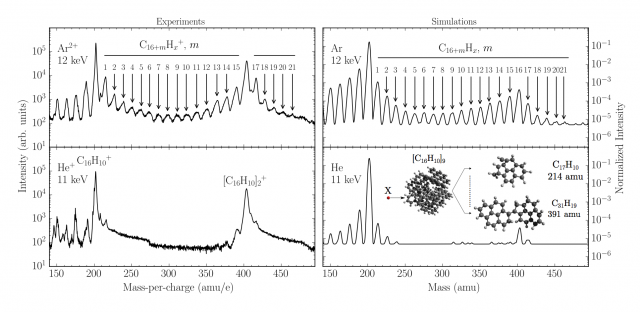
In particular the spectra are scaled such that starting from a pyrene cation (C16H10+) each peak corresponds to the addition of a carbon atom to this molecule till the dimer peak ([C16H10]2+). These simulations, together with higher level of theory tests to validate the method, clearly confirm the reactivity of the fragments and the ability to form larger molecules in consequence of such collisions, as supposed from the experimental results. Similar bond-forming reactions have also been detected in clusters of fullerene molecules, essentially big PAHs closed on themselves. In a study of 22.5 keV
He2+ions colliding with C60clusters, Zettergren et al. detected the formation of covalently bound dumb−bell shaped C118+and C119+molecules 3. Additional computational studies demonstrated that these were produced when the ion knocked out one or two atoms from molecules within the cluster. The remaining C59+or C58+ fragments could then react with neighbouring molecules 4 .
How can all of this be connected to the origins of the Universe? Imagine a cluster of smaller hydrocarbons in the InterStellar Medium, if such collisions happen and provoke the knockout of several atoms with subsequent growth, can ring, benzene-like structures be formed? Can these rings eventually condensate to build PAHs and/or fullerenes? If knockout is at the base of molecular growth in the Universe, this could actually shed some light on complex molecules formation in the Universe and Life birth itself. To this end butadiene (C4H6) clusters were selected first and have been probed in Caen University. Once again experiments are blind to the fine chemistry in the apparatus, but so far simulations have been showing evidences for molecular growth and ring structures formation, even though more data need to be collected and analized 5. These results have been produced by a wide collaboration among different Universities (Caen University, Stockholm University, Porto University and Universidad Autonoma de Madrid) in a nice synergic collaboration between theoreticians and experimentalists. Moreover, collisions with noble gases atoms have been tested on tetraphenyl porphyrin cations in the EIS lab, part of DESIREE facility 6 at Stockholm University to determine if there could be an influence of knockout here as well, which may be also useful to deepen our knowledge on evolution and reactivity of this class of molecules also detected in the ISM, but whose role remains mostly unknown.
In conclusion, recently collected data suggest knockout could play an important role in a wide range of phenomena, including formation of peptides or other small biomolecules in the Universe, and in the processing of DNA or RNA exposed to alpha radiations. Only experiments and calculations can guide us in this dark side of the Universe. So, ”may science be with us!”
References
- Pizzarello, S., G. W. Cooper, G. J. Flynn, edited by D. Lauretta and H. McSween 2006 Meteorites and the Early Solar System II, (Arizona Press, Tucson), p. 625. ↩
- Chen T, Gatchell M, Stockett M H, Alexander J D, Zhang Y, Rousseau P, Domaracka A, Maclot S, Delaunay R, Adoui L, Huber B A, Schlath ̈olter T, Schmidt H T, Cederquist H and Zettergren H 2014, The Journal of Chemical Physics, 140, 224306. ↩
- Seitz F, Zettergren H, Rousseau P, Wang Y, Chen T, Gatchell M, Alexan- der J D, Stockett M H, Rangama J, Chesnel J Y, Capron M, Poully J C, Domaracka A, Mery A, Maclot S, Vizcaino V, Schmidt H T, Adoui L, Alcami M, Tielens A G G M, Martin F, Huber B A and Cederquist H 2013, The Journal of Physical Chemistry Letters, 139, 034309. ↩
- Zettergren H, Rousseau P, Wang Y, Seitz F, Chen T, Gatchell M, Alexan- der J D, Stockett M H, Rangama J, Chesnel J Y, Capron M, Poully J C, Domaracka A, Mery A, Maclot S, Schmidt H T, Adoui L, Alcami M, Tielens A G G M, Martin F, Huber B A and Cederquist H 2013, Physical Review Letters, 110 (18), 185501. ↩
- Article in preparation ↩
- Schmidt H T, Thomas R D, Gatchell M, Rosn S, Reinhed P, Lfgren P, Brnnholm L, Blom M, Bjrkhage M, Bckstrm E, Alexander J D, Leontein S, Hanstorp D, Zettergren H, Liljeby L, Kllberg A, Simonsson A, Hellberg F, Mannervik S, Larsson M, Geppert W D, Rensfelt K G, Danared H, A. Pal, Masuda M, Halldn P, Andler G, Stockett M H, Chen T, Kllersj G, Weimer J, Hansen K, Hartman H, and Cederquist H 2013 Review of Scientific Instruments, 84, 055115. ↩