A route to bulk carbyne
Carbon has four valence electrons. To fill its octet, it requires four additional electrons, which can be obtained through the formation of four covalent bonds. Carbon forms single, double, and triple bonds to achieve a filled octet. As a result, carbon can have a tetrahedral, trigonal planar, or linear geometry, respectively.
A unique feature of carbon is its ability to bond with other carbon atoms to form chains and rings of various lengths. Several other elements have limited ability to form such chains or rings of like atoms, but only carbon does this with more than a few atoms. This featrure results in a wide variety of different carbon allotropes, i.e., forms that the element can be found. Some of them are represented in figure 1.
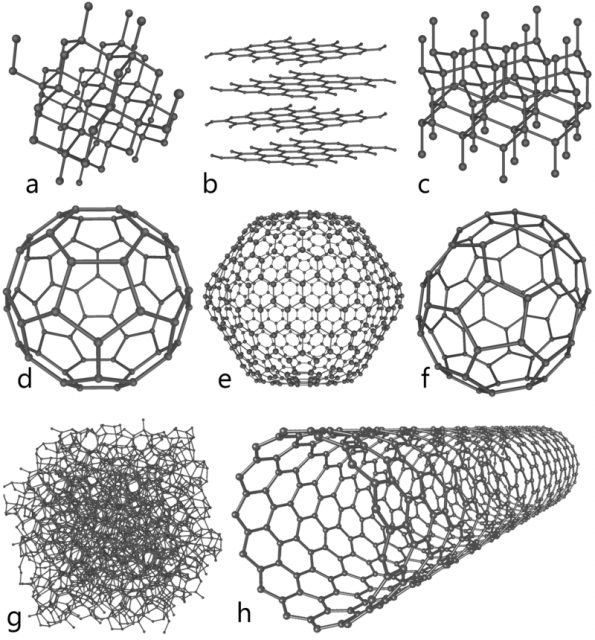
All of these allotropes have been extensively investigated in recent decades. But there is one that is missing, and about which you will find almost no information. Carbyne, an infinite linear carbon chain, formed exclusively by carbon atoms hooked by alternating triple and single bonds, – C ≡ C – C ≡ C – , has attracted much interest and significant controversy since the 1960s.
The identification of carbyne remained questionable for years until the production of polyynes: short linear carbon arrangements with end-capping groups. The longest reported polyynes so far consist of 44 contiguous carbon atoms with alternating single and triple bonds, but the bulk synthesis of very long linear carbon chains (LLCCs) continues to face stumbling blocks.
Little is known about carbyne because of its extreme instability in ambient conditions. In fact, a long standing study postulated the impossibility of preparing this truly one-dimensional (1D) material. The same had been foreseen for graphene on account of its thermodynamic instability, but the recent reports of feasible synthesis routes have inspired revisiting carbyne from both theoretical and experimental points of view.
Carbyne is a very interesting material, to say the least. Carbyne’s theoretically anticipated strength, elastic modulus and stiffness are greater than any known material, including diamond, carbon nanotubes and graphene, and the prospect of new composite materials based on it is extremely appealing.
But, how could we manufacture it? A group of researchers that include MariusWanko, Seymur Cahangirov and Angel Rubio from the Nano-Bio Spectroscopy Group (UPV/EHU), the European Theoretical Spectroscopy Facility, CFM (CSIC-UPV/EHU), and DIPC has developed a method 1 for the bulk production of long acetylenic linear carbon chains protected by a thin double-walled carbon nanotubes. These results establish a route for the bulk production of exceptionally long and stable chains composed of more than 6,000 carbon atoms, representing an elegant forerunner towards the final goal of carbyne’s bulk production.
The experimental research in the production of carbyne has focused heavily on the synthesis of linear carbon chains (LCCs) by different methods, among which, heavy end-capping groups have commonly been used for stabilization. At the same time, single, double and multiwalled (MW) carbon nanotubes have increasingly been used as confining nanoreactors where novel one-dimensional materials can be produced, such as short polyynes, metal nanowires and ultra-narrow graphene.
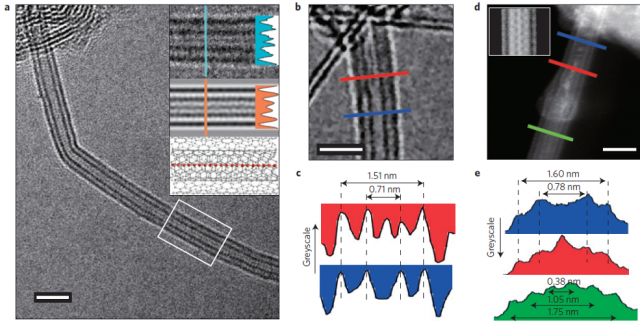
Double-walled carbon nanotubes have facilitated the growth of short carbon chains from high-temperature treatments of buckypaper, as well as carbon nanowires, by promoting the fusion of molecules such as C10H2 or adamantane.
The researchers have sought to use the full potential of narrow-diameter double-walled carbon nanotubes both as nanoreactors and as a tool to encapsulate and protect LLCCs. The procedure uses very high temperature and high vacuum, allowing the bulk realization of the longest LLCCs ever observed, consisting of more than 6,000 contiguous acetylenic carbons inside double-walled carbon nanotubes with ideal diameters.
The high recurrence of LLCCs inside double-walled carbon nanotubes substantiate a truly feasible path towards the bulk-scale formation of carbyne.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
References
- Lei Shi, Philip Rohringer, Kazu Suenaga, Yoshiko Niimi, Jani Kotakoski, Jannik C. Meyer, Herwig Peterlik, MariusWanko, Seymur Cahangirov, Angel Rubio, Zachary J. Lapin, Lukas Novotny, Paola Ayala1, and Thomas Pichler (2016) Confined linear carbon chains as a route to bulk carbyne Nature Materials doi: 10.1038/nmat4617 ↩
1 comment
[…] Conseguir una cadena lineal infinita de átomos de carbono no es precisamente fácil aunque sería un material muy interesante. El DIPC colabora para conseguirlas en A route to bulk carbyne […]