Pain lessons, by the Naked Mole Rat

Nature is one of my favourite places to look for scientific-related answers. As life did start roughly three billion years ago, it has been encountering endless difficulties that needed to be solved in order to prosper. To make things even more complicated, living organisms had to get adapted in multiple different ways because of the variety of natural habitats they had to confront. Fortunately, we can exploit this juncture by looking for animals living in such specific conditions to puzzle out physiological intricacies. To be more illustrative, I would like to mention two examples linked to the study of pain-related physiology.
Back in June 2014, I wrote in Mapping Ignorance about the southern grasshopper mouse living in dry areas from USA and Mexico. The reason why this little mouse attracted scientists’ attention is his unexpected lack of susceptibility to the painful sting from the bark scorpion, arachnid that turns out to be an essential part of the mouse diet. This peculiarity is explained because of the block of the Nav1.8 sodium channel (involved in propagating pain signals to the CNS) by the venomous toxin. Evolution has shaped the grasshopper mouse genome to benefit from an already available (but tricky) source of protein and this has in turn given valuable insight to scientist studying pain propagation.
But perhaps an even more fascinating example is the naked mole-rat, which is a singular eusocial rodent living in tunnels below the soil and showing several interesting attributes, including high resistance to develop tumours, an abnormal longevity or a lack of pain-related behaviour, among others. It did even win the award of “Vertebrate of the Year” back in 2013 (awarded by the journal Science), likely because this small ugly creature conveys some of the most interesting physiological features one can imagine.
The first publication thoroughly addressing the abnormal response of the rodent to painful stimuli dates from 2008 1, although it was already known it lacks neuropeptides important for pain perception on their cutaneous primary afferents 2. The naked mole rat was found to be insensitive to the application of acid and capsaicin algogens, both known to be noxious stimuli 3. There are two ion channels involved in acid response, the acid sensing ion channel family (ASIC) and the TRPV1 ion channel, which I have addressed previously in several posts. In addition, capsaicin is a selective agonist of the TRPV1 channel, thus pointing to impairment in the activation of these channels as the cause. However, the study of isolated primary afferent neurons (DRG) from the rodent did show currents mediated by these channels which were comparable to those of common laboratory mice. Thus, the absence of pain could not be explained by changes on the sensor molecules initiating the nociceptive cascade (the so-called, transducer currents). Looking carefully into more downstream molecules involved in propagation of the nociceptive signals towards the CNS (the machinery from DRG neurons involved in generating action potentials), the authors found the voltage-gated sodium channel Nav1.7 from the naked mole rat was potently inhibited by protons as compared to its laboratory mice counterpart. As this channel is critical to reach the depolarization threshold needed to initiate trains of action potentials, this inhibition prevented the sensory neurons from sending pain-related information to the brain. As opposed to the grasshopper mice, were the inhibition of the Nav1.8 ion channel allows to depolarize the sensory neuron, but halts the propagation of action potentials, in the naked mole rat we do not even reach the necessary threshold to get a first action potential. The result is the same, but the cause is different. Therefore, these pieces of information let us understand in detail the role of each ion channel on each stage of the pain signalling pathway.
Still, the naked mole rat seemed to keep another “painless” secret: the lack of thermal hyperalgesia. In order to protect an injured area after being burned, our body hugely increases the sensitivity of that region so that even light touch will result in pain. Thus, thermal hyperalgesia serves the purpose of keeping the area away from further damage, so that tissue can heal as quick as possible. In a more recent publication, the same group aimed to find an explanation to such lack of physiological response 4.
Once again, TRPV1 plays a key role in thermal hyperalgesia, because the increased sensitivity after the injury is a consequence of the reduction of its temperature-mediated activation threshold (the ion channel gets sensitized). However, they knew from their previous work (and further confirmation from the present one) that nmrTRPV1 (nmr: naked mole rat) is activated in the same fashion as its lab rodent (mice and rat) counterpart, so again the causing molecule had to be another one on the signalling cascade of events. Although it is known that several proinflammatory mediators cause TRPV1 sensitization (bradykinin, histamine or prostaglandin-E2), the authors focused on nerve growth factor (NGF), a neurotrophic factor not only involved in pain, but also in the regulation of the immune system, proliferation or neuronal survival during development. They already had evidence that its sensitizing role over TRPV1 was absent in the naked mole rat, but no explanation to such phenotype was found (1).
They selected the tyrosine kinase receptor TrkA activated upon NGF application to find a potential explanation. NGF binding to a monomeric TrkA receptor leads to dimerization and auto-phosphorylation, initiating the intracellular signalling cascade leading to TRPV1 sensitization, thus TrkA receptor activation is the starting event. Thorough analysis of its DNA sequence (including comparison to several other African mole rat species), led them to conclude the naked mole rat had at least three atypical amino acid variants within its kinase domain (the module involved in phosphorylation and therefore, activation). This observation prompted them to carry a functional approach.
After cloning the rTrkA (from rat, as control) and nmrTrkA receptor along with a chimeric rTrkA receptor bearing the naked mole rat intracellular kinase domain (chTrkA), they transfected oocytes from either Xenopus laevis or human cell lines along with TRPV1. When assessing TRPV1 currents, they found less sensitization fold increase upon NGF application in both nmrTrkA and chTrkA receptors, indicating the nmrTrkA isoform was less efficient sensitizing TRPV1 (Fig 1). An additional quantitative proteomics strategy revealed HEK293 cell line expressing rTrkA had a significant increase in phosphorylation as compared to the chTrkA receptor. Furthermore, this increase in phosphorylation was not just just the result of TrkA phosphorylation, but also downstream kinase pathways such as Erk1 and Erk2.
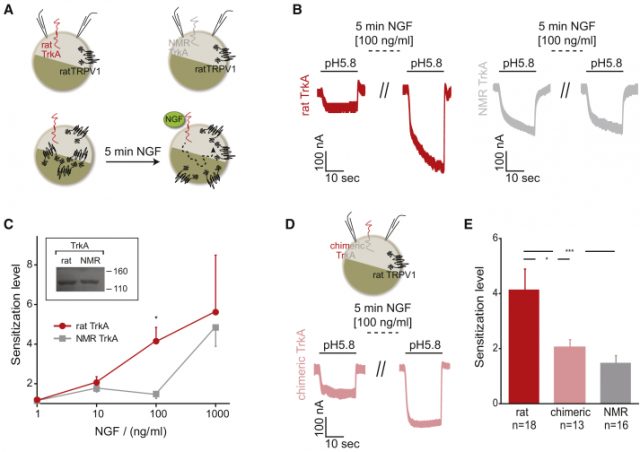
Thus, the lack of heat hyperalgesia is explained by changes in few amino acid substitutions altering the efficiency of key tyrosine phosphorylation within the receptor. More interesting is the fact the species survival is not compromised, given mutations in human TrkA receptor are known to be deleterious, so evolution has found the right balance giving the naked mole rat an adapting advantage without largely affecting other physiological processes. This finding validates the importance of the TrkA receptor in pain and further confirms the molecule as a relevant clinical target for different conditions, such as osteoarthritis to which clinical trials are ongoing with positive results (phase II trial). However, it is important to mention that several companies have previously failed to get approval for anti-NGF molecules, showing once again the difficulty pharma companies face to find the right drug candidates for such applications.
References
- Park TJ, Lu Y, Jüttner R, Smith ES, Hu J, Brand A, Wetzel C, Milenkovic N, Erdmann B, Heppenstall PA, Laurito CE, Wilson SP, Lewin GR. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber). PLoS Biol. 2008 Jan;6(1):e13. doi: 10.1371/journal.pbio.0060013. ↩
- Park TJ, Comer C, Carol A, Lu Y, Hong HS, Rice FL. Somatosensory organization and behavior in naked mole-rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. J Comp Neurol. 2003 Oct 6;465(1):104-20. ↩
- Smith ES, Omerbašić D, Lechner SG, Anirudhan G, Lapatsina L, Lewin GR. The molecular basis of acid insensitivity in the African naked mole-rat. Science. 2011 Dec 16;334(6062):1557-60. doi: 10.1126/science.1213760. ↩
- Omerbašić D, Smith ES, Moroni M, Homfeld J, Eigenbrod O, Bennett NC, Reznick J, Faulkes CG, Selbach M, Lewin GR. Hypofunctional TrkA Accounts for the Absence of Pain Sensitization in the African Naked Mole-Rat. Cell Rep. 2016 Oct 11;17(3):748-758. doi: 10.1016/j.celrep.2016.09.035. ↩
1 comment
[…] La rata topo desnuda carece de un comportamiento asociado al dolor, lo que la convierte en una fuente muy interesante de información sobre la fisiología del dolor. Sergio Laínez en Pain lessons, by the Naked Mole Rat […]