Gene therapy is back: The X-ALD case
Author: Pablo Ranea is a postdoctoral scientist at Bellvitge Biomedical Research Institute – IDIBELL
Sometimes, scientists give very good news, and in 2017 we have had one of these occasions. Boys suffering from X-linked adrenoleukodystrophy (X-ALD), a devastating neurodegenerative disease, have been cured with gene therapy. The STARBEAM study was performed by an international team headed by David A. Williams from the Boston Children’s Hospital in Massachusetts (USA), and the results of the study have been recently made public. After two years, 15 out of the 17 boys enrolled in the study do not present major functional disabilities characteristic of the disease1. These data represent one of the most important advances in the history of X-ALD, after the discovery of the gene involved and the implantation of bone marrow transplantation in the early 90s.
The study consisted in the genetic modification of the patient’s own hematopoietic stem cells, by the transduction of the correct copy of ABCD1 (the gene mutated in X-ALD) with a lentiviral vector (a virus that carries the correct copy of the gene and integrates into the host) (Figure 1). Genetically modified cells would lead to the production of functional adrenoleukodystrophy protein (ALDP). This protein is involved in the breakdown of very-long-chain fatty acids (VLCFA) in the peroxisome. In X-ALD, the accumulation of VLCFA (fatty acids with 22 or more carbons) results in neurodegeneration by mechanisms that are not fully understood yet. “Adreno” refers to the adrenal glands, “leuko”’ refers to the white matter of the brain, and “dystrophy” indicates abnormal growth or development. 35% of the X-ALD patients suffer the childhood cerebral form of the disease (ccALD), which is the most severe. Boys with ccALD present inflammatory cerebral demyelination and most of them die in few years. The only therapeutic option for them up to date was allogeneic hematopoietic stem cell transplantation, performed by bone marrow transplantation from a compatible donor. This therapy comes with limitations like the risks of graft failure and graft-versus-host disease, together with the possible lack of a matched donor before the disease progresses.
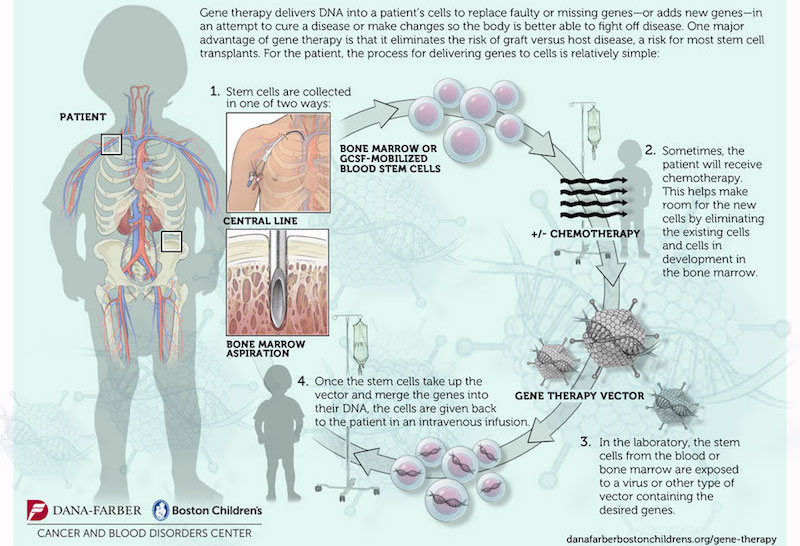
In this context, this gene therapy study represents a light at the end of the tunnel. To select the patients for gene therapy, it is mandatory that they do not present an advanced stage of the disease. By magnetic resonance imaging (MRI), a score from 0 to 34 (Loes score, higher scores indicate an increased magnitude of the lesions on MRI) was given to the candidates, and only those with a score between 0.5 and 9 were selected. To measure neurologic impairments, a specific scale from 0 to 25 was used, with higher scores indicating more severe deficits (more details in the study).
The results of the study are encouraging. ALDP (the protein encoded by ABCD1 gene) was expressed in patients’ blood cells after the transfusion of the stem cells. Regarding the clinical outcome, 15 patients remained in a neurologic score below 2 (remember the range between 0 and 25, with higher scores indicating more severe deficits). This means that they did not present any abnormalities characteristics of X-ALD. 2 patients died during the study, one of them was withdrawn from the study and was subjected to bone marrow transplantation but died due to complications in the procedure. In the other one, the disease progressed quickly and died 2 years after the gene therapy. Curiously, these adverse events in these two patients correlated with a lower viral load in the drug product compared with the other patients. The sample size is low, usual in rare diseases studies, so they could not confirm a significant difference, but this correlation suggests that those patients did not receive enough copies of the ABCD1 gene to halt the inflammation. The Loes score (the extent of the lesions by MRI) also remained stable in 11 out of 17 patients. The patient who died due to a fast progression of the disease had the second highest Loes score (Loes score = 7), which also points to the importance to start the treatments in an early phase of the disease (see the Study for more details about every patient). Another important parameter that was measured is gadolinium enhancement, a chemical compound that improves the contrast of the MRI, and which was present in the initial MRI of all the patients. This media injected into the patients consists of a mix of gadolinium ions with a chelating agent that prevents the toxicity of the gadolinium. Gadolinium enhancement is typically observed near the leading edge of active demyelination and, when present, strongly predicts disease progression. At the end of this study, the enhancement was not present in most of them, and in the remaining patients, the enhancement was less extensive.
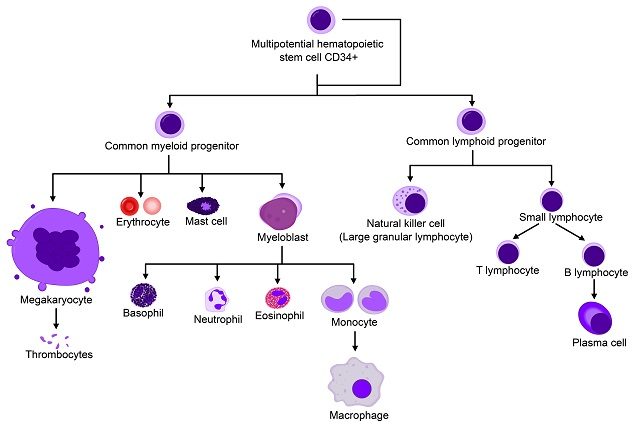
This study is based on a proof-of-principle initial study published in 2010 in which two Spanish boys received the transfusion of genetically-modified hematopoietic stem cells, and after two years, the disease had not progressed and they remained stable 2. Gene therapy has regained a central scene in biomedicine and the pharmaceutical companies after famous pitfalls that happened in the 90s. 5 out of 20 patients from one of the first gene therapy studies developed leukaemia due to the integration of the vector near proto-oncogenes, which resulted in the activation of their expression 3. One of the key changes has been the switch from retroviral to lentiviral vectors, which seem to possess less risk of mutagenesis. Hematopoietic stem cells are ideal for these approach as they are long-lived and multipotent. Moreover, they can be accessed for ex vivo manipulation, genetic modification and re-administration to the patient by an intravenous transfusion. CD34 is a glycoprotein and an antigen expressed in hematopoietic stem cells. Patient’s own CD34+ stem cells are selected for the manipulation and transfusion, which ideally would lead to the genetic correction in the hematopoietic lineage (Figure 2).
However, gene therapy does not prevent the appearance of myelopathy, the main component of the X-ALD adult phenotype, which is adrenomyeloneuropathy (AMN) 4. AMN patients present peripheral axonopathy with the spinal cord as the main affected tissue. Gene therapy halts cerebral inflammation but it does not normalize the levels of VLCFA (the diagnostic biomarker of the disease), so the toxic effects of VLCFA eventually lead to the onset of AMN in adulthood. That is why it is so important to investigate the mechanism associated with VLCFA accumulation and to develop therapies for AMN patients, who cannot benefit from gene therapy or bone marrow transplantation. In our group, we are investigating the pathophysiology of X-ALD, by studying the toxic effects of VLCFA in the mouse model of the disease (Abcd1 knockout mouse) and in skin fibroblasts from X-ALD patients. We are developing therapies for AMN patients after understanding the pathophysiology. For example, we discovered that oxidative stress and mitochondrial dysfunction, among other dysregulated pathways, are early hallmarks of X-ALD 56, and we are evaluating an antioxidant cocktail in a clinical trial with AMN patients after showing the efficacy of this cocktail in the mouse model 7.
Being a scientist is hard, but these kind of results are amazing stimuli to keep doing science.
More info:
http://www.neurometabolic-lab.org
References
- Eichler, F., Duncan, C., Musolino, P. L., Orchard, P. J., De Oliveira, S., Thrasher, A. J., … Williams, D. A. (2017). Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. New England Journal of Medicine, 377(17), 1630–1638. doi: 10.1056/NEJMoa1700554 ↩
- Cartier, N., Hacein-Bey-Abina, S., Bartholomae, C. C., Veres, G., Schmidt, M., Kutschera, I., … Aubourg, P. (2009). Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science, 326(5954), 818–823. 10.1126/science.1171242 ↩
- Hacein-Bey-Abina, S., Garrigue, A., Wang, G. P., Soulier, J., Lim, A., Morillon, E., … Cavazzana-Calvo, M. (2008). Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. The Journal of Clinical Investigation, 118(9), 3132–42. doi: 10.1172/JCI35700 ↩
- van Geel, B. M., Poll-The, B. T., Verrips, A., Boelens, J.-J., Kemp, S., & Engelen, M. (2015). Hematopoietic cell transplantation does not prevent myelopathy in X-linked adrenoleukodystrophy: a retrospective study. Journal of Inherited Metabolic Disease, 38(2), 359–361. doi: 10.1007/s10545-014-9797-1 ↩
- Fourcade, S., Lopez-Erauskin, J., Galino, J., Duval, C., Naudi, A., Jove, M., … Pujol, A. (2008). Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum Mol Genet, 17(12), 1762–1773. doi: 10.1093/hmg/ddn085 ↩
- Lopez-Erauskin, J., Galino, J., Ruiz, M., Cuezva, J. M., Fabregat, I., Cacabelos, D., … Pujol, A. (2013). Impaired mitochondrial oxidative phosphorylation in the peroxisomal disease X-linked adrenoleukodystrophy. Hum Mol Genet, 22(16), 3296–3305. doi: 10.1093/hmg/ddt186 ↩
- Lopez-Erauskin, J., Fourcade, S., Galino, J., Ruiz, M., Schluter, A., Naudi, A., … Pujol, A. (2011). Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann Neurol, 70(1), 84–92. doi: 10.1002/ana.22363 ↩