Towards a vaccine against HIV
Towards a vaccine against HIV
In 1981 the Center for Disease Control and Prevention (CDC) of the United States reported the existence of anunexplained pneumonia and Kaposi’s sarcoma by Pneumocystis jiroveci in previously healthy homosexual men from Los Angeles and New York. This represented the first time that the Acquired Immunodeficiency Syndrome (AIDS) was identified. Subsequently, cases of AIDS increased in individuals addicted to parenterally inoculated drugs.
Two years later, in 1983, the agent associated with the disease was identified by Luc Montagnier and in 1984, nearly simultaneously, by Robert Gallo. For this discovery, Luc Montagnier was awarded with the Nobel Prize in Medicine in 2008.
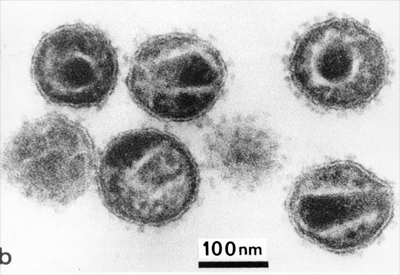
HIV, the causative agent of AIDS, is a Lentivirus belonging to the Retroviridaefamily. Within the HIV we find two types: HIV-1 and HIV-2, being HIV-1 the most frequent cause of HIV disease worldwide 1.
HIV is an icosahedral, single-stranded, positive-sense, enveloped RNA virus whose main characteristic is that encodes for an enzyme with reverse transcriptase activity. This enzyme allows this virus to convert its RNA to DNA for integration into the genome of infected cells.
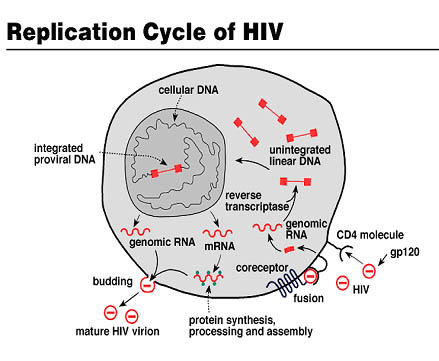
In addition, this virus includes spikes on its surface: glycoproteins gp 120 and gp 41, which are important for the virus at the early stage of the infectious cycle, being gp 120 responsible for recognizing the cellular receptor CD4. This cell receptor is present on cytotoxic T lymphocytes, which are the target immune system cells of this infectious agent.

After recognition between gp120 and the cellular receptor CD4 (the tropism also includes recognition of chemokine coreceptors), fusion of the viral envelope with the cell membrane occurs and the HIV capsid is released into the cell. Subsequently, the viral genome is integrated into the host genome after retrotranscription by means of theviral reverse transcriptase.
At this stage, virions will be produced into the host due to the cell biochemical machinery. This phase, in which the HIV genome remains integrated in the genome of CD4+ T lymphocytes and new produced virions remain protected inside the host, is called lysogenic phase.
Afterwards, when the lytic phasebegins,viruses exit the host. In this stage, the virus destroys the cell to go elsewhere in search of other cells to infect. During this phase the organism tries to replenish dead cells. Diagnostically, the disease known as AIDS is considered when CD4+ T lymphocytes population of infected individuals decline drastically (less than 200/uL).
Due to CD4+ T lymphocytes constitute one of the major defence mechanisms of the body, with its supression HIV leaves us prone to be infected by a wide variety of opportunistic pathogens.
Since its discovery (more than thirty years ago) and even today there is still no effective vaccine against HIV/AIDS. And we cannot say that there have not been money and effort invested in such a research, just the opposite. However, all efforts have been in vain…or not?
Definitely not.
Note that during the research in the field of HIV, we obtained significant advances. Initially AIDS was a fatal disease. However, thanks to antiretroviral treatments, it has become a chronic disease. A breakthrough achieved by research that has allowed patients to live with a high quality of life.
It must be taken into account that HIV-1 is a virus with high genetic variability and replication kinetics, allowing it to easily adapt to changes in its environment. This, in turn, enables the virus to become resistant to antiretroviral drugs after a prolonged exposure to them. For that reason, despite the great progress made in terms of therapy with different drugs, the search for a vaccine remains a work to be done.
Several approaches to the final vaccine.
Since its discovery, the optimism about the development of a vaccine has always been overstated. No wonder every so often you can read articles in which it was described an antibody, peptide or substance that promised to be the ultimate preventive or therapeutic vaccine.
We must be more careful in our forecasts and away from false hopes about this issue because there are many people who have great expectations in this field. What they need are results.
Today, more than thirty years after the discovery of HIV, the scientific community is still fighting against this serious public health problem that has caused nearly 30 million deaths worldwide.
The biggest “success” in the vaccine development was a vaccine that used a modified poxvirus with the gene for protein gp120. This vaccine, the only of this kind that has reached Phase III clinicaltesting, provided a 31%protection in some populations, which is obviously insufficient for large scale use.
But why is it so difficult to get a definitive vaccine?
A research carried out by scientists at Oregon Health & Science University (published in Nature Medicine) 2 explains that the main problem is the graduation of the immune response generated.
When we speak of preventive vaccines, traditionally eliciting an immune response to prevent HIV infection has been achieved using two different techniques: the inoculation of attenuated whole virus (the virus is inactivated so that the infection is not produced) and inoculation of the more immunogenic portions of the virus (mainly surface protein generally recognized by the cellular receptors).
In both cases we find priblems. In the case of attenuated whole virus inoculation because there is no certainty that the inactivated virus is not able to cause the infection in vaccinated people. In the case of inoculation of viral proteins the immune response generated is insufficient. However, the most interesting aspect of this work is the conclusion they reached: the use of an intermediate mechanism, i.e., inoculating a whole virus that is safe for humans which contains the HIV proteins of interest.
In this case, there are several candidatessuch as adenovirus (ADV), cytomegalovirus (CMV) or vaccinia virus (VV). The latter was featured in 2011 in a study 3carried out by the group of Dr. Mariano Esteban, a researcher at the National Center for Biotechnology (CNB) of the National Research Council (CSIC) in Spain.
In this promising trial, the researchers managed to pass Phase I clinical testing as a vaccine against HIV. In this study, they used the modified vaccinia virus Ankara (MVA) [footnoye]Gómez CE, Nájera JL, Perdiguero B, García-Arriaza J, Sorzano CO, Jiménez V, González-Sanz R, Jiménez JL, Muñoz-Fernández MA, López Bernaldo de Quirós JC, Guardo AC, García F, Gatell JM, Plana M, Esteban M. The HIV/AIDS vaccine candidate MVA-B administered as a single immunogen in humans triggers robust, polyfunctional, and selective effector memory T cell responses to HIV-1 antigens. J Virol. 2011 Nov;85(21):11468-78. doi: 10.1128/JVI.05165-11.[/footnote]. Four genes of HIV subtype B were incorporated to its genome. This MVA-B was inoculated to 24 volunteers, showing optimal security with regard to their application as well as an efficient generation of immune response in 95% of patients who received three doses of vaccine, compared to placebo patients inoculated.
This modified virus has several features that make it a potential candidate for a viable vaccine. It not only generates the synthesis of antibodies capable of neutralizing the virus but it is also able to increase the number of lymphocytes responsible for recognizing and destroying them.
Despite the good results of this vaccine, which response was found to be up to six times greater than a similar vaccine tested in a study in Thailand, we have to be cautious and wait to see what happens with this vaccine in the coming years.
As for vaccines that prevent infection a recently published work 4, developed by a group of scientists from the National Centre for Scientific Research (CNRS) and the University Paris-Sud, describe a gel containing receptor molecules that interact with the surface proteins of the virus. When viruses contact with the gel they are neutralized. The interaction between receptor molecules present in the gel and the viral receptors prevents the contact between viruses with their counterparts of the host cells. In this way the virus is exposed to the body’s defenses, which can eliminate it. The results with this product proved to be highly effective inhibiting transmission between primates, achieving that five of the six females used in the experiment being non-infected.
Finally, it is worth emphasizing the work done by the group of Dr. Josep Maria Gatell, from the Hospital Clinic of Barcelona in Spain. In this study 5, published in the Science Translational Medicine Journal, they describe a potential therapeutic vaccine against HIV.
Basically, the mechanism they describe in this paper is to extract patient’s dendritic cells. These cells are infected in vitro with the same kind of inactivated HIV affecting the patient. Infected cells are re-inoculated in the organism. At the moment in which infected cells enter into the lymph nodes, these cells activate the immune system against the virus.
This test was performed in 36 patients who continued antiretroviral therapy. They showed a reduction of more than three times in viral load in 95% of those infected, while in the control patients who received a placebo this reduction did not occur. However, the positive effects of the vaccine begin to decline after the twelfth week, disappearing after a year of implementation. For that reason, the main interest of this vaccine would focus on complementary use with antiretroviral treatment, with the aim of prevent HIV to become resistant to antiretroviral drugs used in treatment.
However, the goal of researchers remains to find a vaccine which is capable of controlling the replication of HIV indefinitely. It would provide a viable alternative to antiviral cocktails, which lasts for life at a high cost, in addition to the inconvenience for patients.
Acknowledgments
I want to thank Jose Antonio López Guerrero for his helpful comments on this article
References
- Alfonso Paño Lalana. El SIDA como enfermedad zoonótica. Journal of Feelsynapsis (JoF). ISSN: 2254-3651. 2012 (2): 12-15 ↩
- Nancy L. Haigwood and Vanessa M. Hirsch. Blocking and tackling HIV. Nature Medicine 15, 841 – 842 (2009) doi:10.1038/nm0809-841 ↩
- García F, Bernaldo de Quirós JC, Gómez CE, Perdiguero B, Nájera JL, Jiménez V, García-Arriaza J, Guardo AC, Pérez I, Díaz-Brito V, Conde MS, González N, Alvarez A, Alcamí J, Jiménez JL, Pich J, Arnaiz JA, Maleno MJ, León A, Muñoz-Fernández MA, Liljeström P, Weber J, Pantaleo G, Gatell JM, Plana M, Esteban M. Safety and immunogenicity of a modified pox vector-based HIV/AIDS vaccine candidate expressing Env, Gag, Pol and Nef proteins of HIV-1 subtype B (MVA-B) in healthy HIV-1-uninfected volunteers: A phase I clinical trial (RISVAC02). Vaccine. 2011 Oct 26;29(46):8309-16. doi: 10.1016/j.vaccine.2011.08.098 ↩
- Dereuddre-Bosquet N, Morellato-Castillo L, Brouwers J, Augustijns P, Bouchemal K, Ponchel G, Ramos OH, Herrera C, Stefanidou M, Shattock R, Heyndrickx L, Vanham G, Kessler P, Le Grand R, Martin L. MiniCD4 Microbicide Prevents HIV Infection of Human Mucosal Explants and Vaginal Transmission of SHIV(162P3) in Cynomolgus Macaques. PLoS Pathog. 2012 Dec;8(12):e1003071. doi: 10.1371/journal.ppat.1003071 ↩
- García F, Climent N, Guardo AC, Gil C, León A, Autran B, Lifson JD, Martínez-Picado J, Dalmau J, Clotet B, Gatell JM, Plana M, Gallart T; For the DCV2/MANON07-ORVACS Study Group. A Dendritic Cell-Based Vaccine Elicits T Cell Responses Associated with Control of HIV-1 Replication. Sci Transl Med. 2013 Jan 2;5(166):166ra2. ↩