Nanotechnology inspired by nature
Nanotechnology inspired by nature
Author:
Leire Gartzia Rivero got a Chemistry degree and completed her Master studies in “New Materials” at the University of the Basque Country. She holds a Ph.D. in Materials Science and Technology obtained at the same university, in the Molecular Spectroscopy Laboratory, where she focused on the spectroscopic characterization of photoactive nanomaterials based on energy transfer processes. She is currently a postdoctoral researcher at the Institute of Molecular Sciences (ISM), University of Bordeaux, supported by the Postdoctoral Program of the Basque Government for the improvement of researchers. She is investigating the self-assembly of organic molecules towards the obtention of fluorescent nano-objects using advanced microscopy techniques.
It’s not so long ago that scientists of different fields all around the world realized how perfect and efficient the processes in nature are. So, why not take inspiration from them to create new nanotechnology? In fact, one of the main temptations of a chemist consists of mimicking functions and mechanisms present in nature and use them as a way to guarantee technological progress. Among the broad range of choices reachable for the scientist, we will focus on the attempt to develop new fluorescent nanomaterials taking inspiration from plant antenna systems 1.
These photosynthetic microorganisms are present in specific areas of the plant cells, called chloroplasts, and consist of thousands of chlorophyll molecules embedded in a protein matrix (Figure 1). The main characteristics of these systems are their antenna function, which enables them to harvest solar energy in a broad spectral range and transport it by means of multiple and efficient energy transfer processes to a specific reaction centre, where it is finally turned into chemical energy.
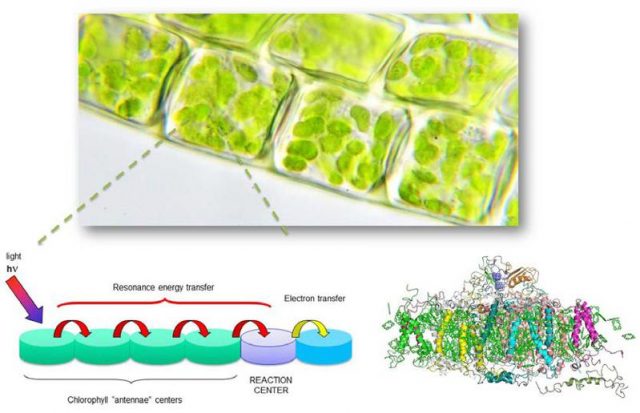
It is a well-known fact that solar radiation is made up of many colours (blue, green, yellow, red, etc.), as borne out by the broad range of colours present in the rainbow. The aim of artificial antenna systems is to capture the broadest light range possible so that it can then be efficiently turned into electrical energy (photovoltaic devices) or converted exclusively into red light, so useful in photonic and biomedical applications. In this respect, and with the aim of coming up with bioinspired artificial antenna systems, the Group of Molecular Spectroscopy at UPV/EHU has developed an assortment of new dyes and photoactive nanomaterials. These systems harvest a broad interval of chromatic radiation and convert it into a light of different energies, providing red-edge emission or alternatively white-light emission, depending on the desired application (Figure 2).
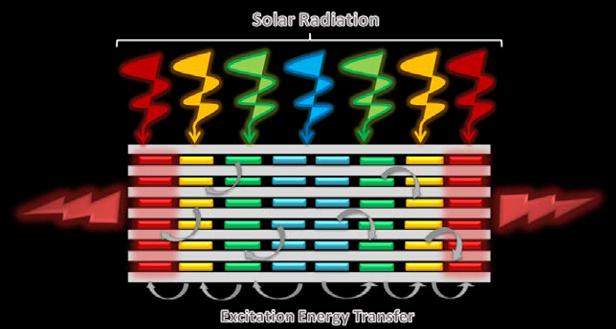
Energy donor and acceptor molecules coexist in these photoactive dyes and nanomaterials. The former are highly photostable fluorescent molecules and are responsible for absorbing the light and transfer it to the acceptor species, which will then emit light. This strategy allows reducing the limitations inherent in the red dyes, which are characterized by reduced light absorption and low photostability, and offers a great advantage in photonic and biophotonic applications, as they allow the photostability of the system and detection sensitivity to be improved.
Three different alternatives have been chosen to develop these antenna systems (Figure 3): two of them are based on the encapsulation of fluorescent dyes in either inorganic or organic hosts, and the other one in the assembly of different dyes into a single molecular structure. The protein matrix of the natural systems has been replaced by synthetic hosts of nanometric dimensions, which protect the dyes and provide a significant arrangement that will help to make the energy transfer processes viable and efficient. Furthermore, with respect to the photoactive part, which is responsible for interacting with the light, the chlorophyll molecules have been replaced by fluorescent molecules many of which have been customized à la carte.

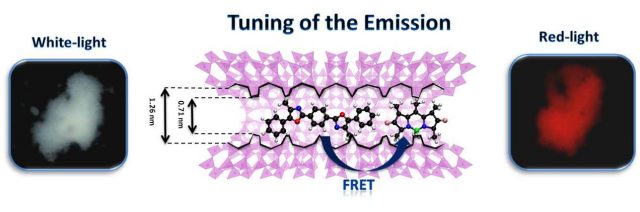
In the first of the alternatives, the chosen solid matrix to encapsulate the fluorescent dyes is a crystalline aluminosilicate known as LTL Zeolite, characterized by the fact that it has unidimensional channels and a suitable pore size (7Å) in which the molecules fit like a glove. This produces a highly ordered nanomaterial that allows the light emission to be modulated to produce a red or white light depending on the control exerted on the efficiency of the energy transfer process (Figure 4) 2. This chameleon-like property turns them into materials capable of generating new light emitting diodes (LEDs), featuring white-light emitting diodes (WLED), which are so useful in lighting technologies such as liquid crystal displays (LCD).
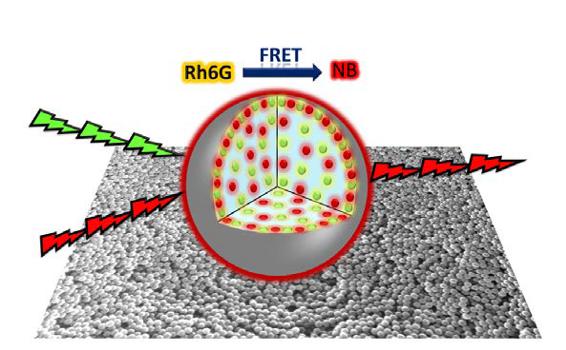
The other chosen matrix to host dyes consists of polymer nanoparticles capable of holding extremely high dye concentrations inside them without it becoming aggregated (Figure 5). Confining the dyes reduces the photodegradation processes, considerably increases their useful service life and encourages the transfer of energy, which enables not only to obtain an antenna system, but also tunable red laser radiation that is efficient and long-lasting in stable aqueous suspensions 3.
On the other hand, the antenna systems can be also made up solely of organic molecules in which the energy donor and acceptor species are linked by a spacer ensuring short intermolecular distances, thus achieving efficiencies in the energy transfer processes of practically 100%.
![Figure 6. Molecular antenna system: blue, green and red emitting dyes covalently linked to allow a fast and highly efficient energy transfer that ends up in near-infrared laser emitting (Picture taken of Ref [4]).](https://mappingignorance.org/app/uploads/2016/05/Figure-6.jpg)
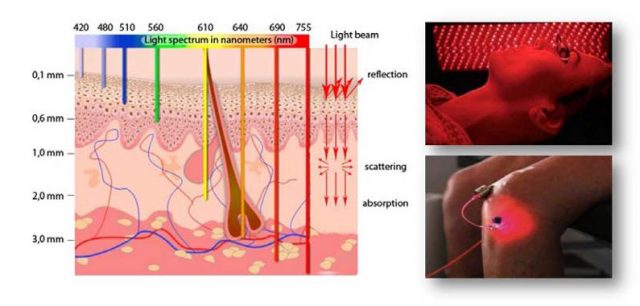
This has meant a great improvement in the harvesting of light across the visible spectrum, leading to exclusively stable bright red which means they are highly recommended as active hosts for tunable lasers in the zone close to the infrared. The main interest in this wavelength is its great tissue penetration capacity, a key in photodynamic therapy with uses in ophthalmology and dermatology, as well as in cancer treatment (Figure 7). Besides, they also deserve special interest in biophysics science and technology due to the reduced background interferences, necessary for bioimaging and sensing applications, since the emission can be monitored far away from the excitation wavelength.
References
- L. Gartzia-Rivero, J. Bañuelos & I. López-Arbeloa (2015), “Excitation energy transfer in artificial antennas: from photoactive materials to molecular assemblies,” International Reviews in Physical Chemistry; vol. 34, nº 4, pp. 515-556. doi: 10.1080/0144235X.2015.1075279 ↩
- L. Gartzia-Rivero, J. Bañuelos-Prieto, V. Martínez-Martínez & I. López Arbeloa (2012), “Versatile Photoactive Materials Based on Zeolite L Doped with Laser Dyes,” ChemPlusChem, vol. 77, nº 1, pp 61-70. doi: 10.1002/cplu.201100020 ↩
- L. Cerdán, E. Enciso, V. Martín, J. Bañuelos, I. López-Arbeloa, A. Costela & I. García-Moreno (2012), “FRET-assisted laser emission in colloidal suspensions of dye-doped latex nanoparticles,” Nature Photonics, vol. 6, pp. 621-626. ↩