Rewiring the brain: How blocking adenosine boosts stroke healing
Rewiring the brain: How blocking adenosine boosts stroke healing
Each year, millions of people experience an ischaemic stroke, a condition where a blood clot blocks the flow of oxygen and nutrients to part of the brain. Even when doctors act quickly to remove the clot, many survivors struggle with long-term challenges like difficulty moving, remembering, or managing emotions. While treatments like clot-dissolving drugs or mechanical clot removal can save brain tissue in the critical first hours, there’s little available to help the brain heal in the weeks that follow. A recent study 1 explores a new approach to improve recovery by gently encouraging the brain’s natural repair process, focusing on the adenosine A1 receptor.
Why adenosine is important in a struggling brain
Adenosine is a small molecule familiar to many as part of ATP, the energy source that powers our cells. When a stroke disrupts blood flow, the brain’s energy supply falters, causing ATP to break down and adenosine levels to spike. This molecule then interacts with proteins on cell surfaces called adenosine receptors, which act like switches to control brain activity and blood flow. One of these, the A1 receptor, typically calms brain cells and reduces energy use during the chaotic minutes after a stroke, which seems protective at first. However, keeping this receptor active for too long may hinder the brain’s ability to adapt and repair. It can reduce the brain’s flexibility and favour the growth of scar-forming cells over new nerve cells. This led researchers to hypothesize that blocking the A1 receptor after the initial crisis could encourage the brain to produce more neurons and less scar tissue, potentially aiding recovery.
A daily block of the adenosine receptor
To test this, the researchers caused a stroke in rats and mice by temporarily blocking a major brain artery, mimicking a common type of human stroke. A few hours after restoring blood flow, they began daily injections of a compound called DPCPX, which specifically blocks the A1 receptor. These injections continued for up to four weeks, a period when the brain naturally tries to repair itself by generating new cells. The team then examined three key outcomes: how many new cells were being made, what types of cells they became, and whether the animals showed improved movement or memory.
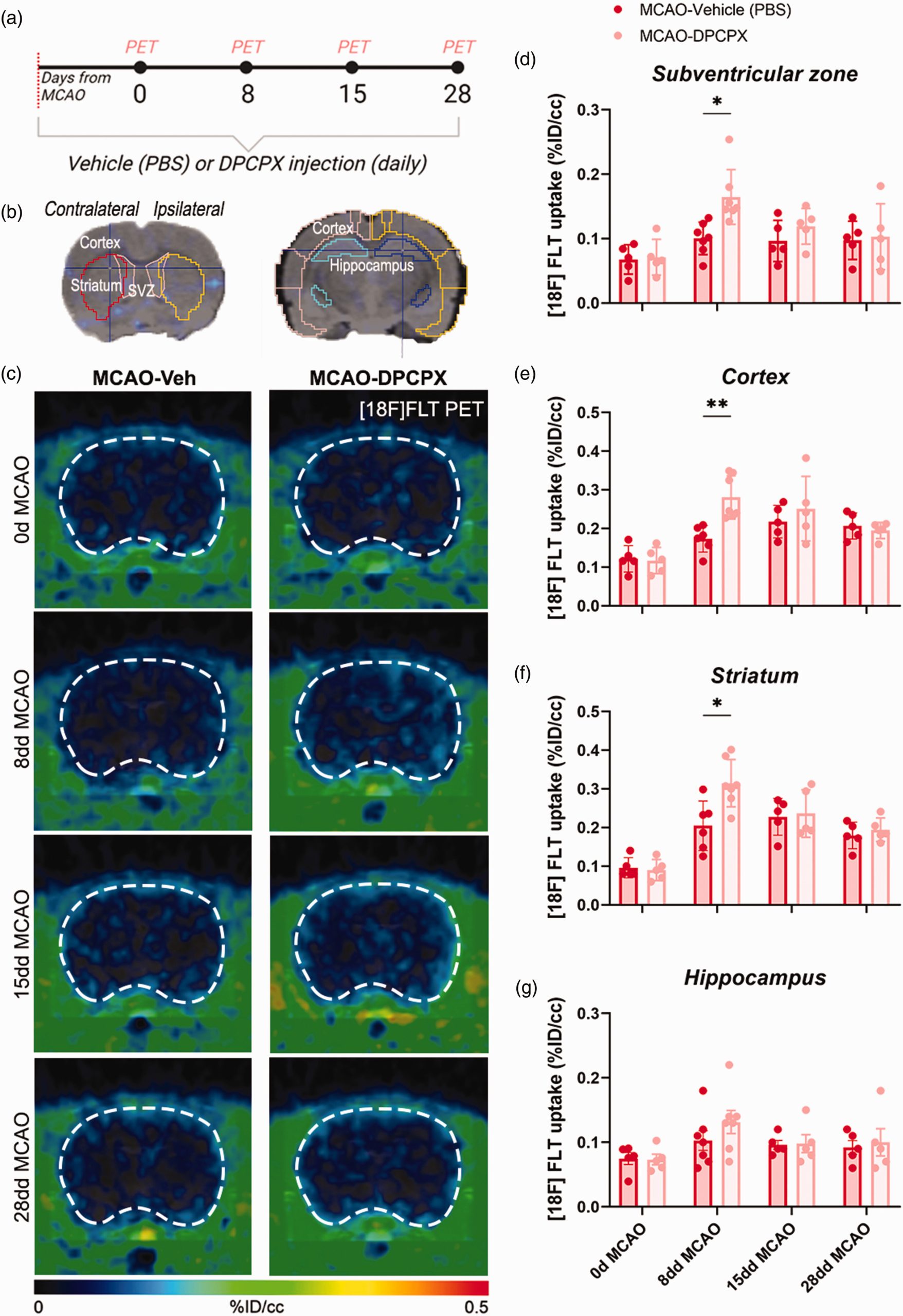
Watching new cells grow in real time
Counting new cells deep in the brain is challenging, but the team used two clever methods. First, they injected a radioactive tracer called [18F]FLT, which gets absorbed by dividing cells. Using a PET scan, they saw brighter signals in the brains of DPCPX-treated animals, especially in the subventricular zone—a key area where stem-like cells live—as well as in damaged regions like the cortex and striatum. This suggested more cells were dividing. To confirm, they used microscopes to examine brain tissue stained with markers like BrdU and Ki67, which highlight dividing cells. These tests showed that DPCPX-treated brains had far more dividing cells than untreated ones. Importantly, stains for proteins like doublecortin and NeuN, which mark young and mature neurons, revealed that more of these new cells became neurons rather than scar-forming glial cells, as seen by lower levels of a marker called thrombospondin-4.
Better movement and memory
The real test was whether these cellular changes improved how the animals functioned. In tasks like balancing on a rotating rod or climbing a pole, DPCPX-treated mice regained coordination and strength faster than untreated ones, often performing as well as healthy animals within two weeks. In a memory test where mice explored new objects, treated animals showed better short-term memory and curiosity, indicating sharper cognitive abilities. They also had higher survival rates and smaller areas of brain damage after four weeks, suggesting the treatment helped both repair and protect the brain.
What this means and what’s next
The findings suggest that blocking the A1 receptor with a daily, low-dose treatment can push the brain toward a more regenerative state. Stem-like cells in the subventricular zone multiply, travel to damaged areas, and turn into neurons that help rewire the brain’s networks, leading to better movement and memory. This approach tackles both the physical damage (by reducing scarring) and the functional recovery (by improving brain connections).
However, there are limitations to consider. The study used young, healthy rodents, but human stroke patients often have conditions like high blood pressure or diabetes, which could affect results. DPCPX isn’t approved for human use, and since A1 receptors are found in many organs, side effects need careful study. Timing is also critical: earlier research showed that blocking A1 receptors too soon after a stroke can worsen outcomes, likely because the receptor’s initial calming effect is beneficial. This study started treatment after blood flow was restored, indicating a specific window for effective therapy.
A new path forward
This research offers an exciting possibility: instead of transplanting cells from outside, we can harness the brain’s own repair system. If future studies confirm that A1 receptor blockers are safe and effective, they could become part of a two-step stroke treatment—clear the clot first, then help the brain rebuild. For stroke survivors facing the slow, often frustrating road to recovery, this approach could offer new hope for regaining lost abilities.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Maria Ardaya, Monica Benito-Munoz , Esther Rubio-Lopez, Maider Garbizu, Laura Aguado, Naroa Mocha-Munoz, Leyre Iglesias, Unai Aldutzin, Carlos Matute, Federico N Soria, Vanessa Gomez-Vallejo, Aitzol García-Etxarri, Jordi Llop, Fabio Cavaliere and Abraham Martín (2025) Chronic treatment with adenosine A1 receptor antagonist promotes neurogenesis and improves outcome after cerebral ischemia Journal of Cerebral Blood Flow & Metabolism doi:10.1177/0271678X251345294 ↩