One pore to rule them all: Bax assembles into rings and arcs in apoptotic mitochondria
One pore to rule them all: Bax assembles into rings and arcs in apoptotic mitochondria
Author:
Raquel Salvador-Gallego studied Biochemistry at the University of Zaragoza, then moved to Heidelberg (Germany) to pursue her Ph.D. in the Membrane Biophysics group headed by Ana García-Sáez at the German Cancer Research Center and the Max Planck Institute for Intelligent Systems. They moved to the University of Tübingen, where she is about to submit her thesis.
Although it may sound illogical, destruction of cells is as essential as their creation in the course of proper formation, growth and development of multi-cellular organisms. For this reason, cells program deliberately their suicide through a very controlled process, named apoptosis (from the Greek, “fall off”). Our research group at the University of Tübingen, Germany works to unravel the precise mechanisms behind this vital process. This type of programmed cell death is crucial for proper organ shape and for the development of the nervous system. For example, our fingers and toes are connected by a webbing before we are born until the cells between them undergo apoptosis. In our brain, the cells that are not needed for good synaptic connections also have to die. Moreover, cells kill themselves off when they sense a potential threat to the organism, such as a virus infection or DNA damage.
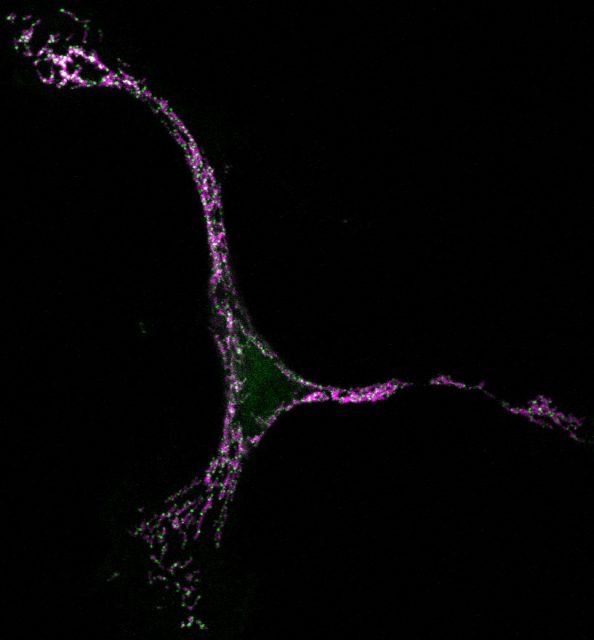
Unfortunately, this is not a perfect process and the imbalance between cell proliferation and death carries fatal consequences for our organs. Excessive cell death is associated with the occurrence of strokes, heart attacks, or diseases such as Alzheimer´s and Parkinson´s. However, if something fails and apoptosis is not completed, the cells grow and accumulate massively leading to autoimmune diseases or cancer1.
The Bcl-2 family of proteins is the main orchestrators of apoptosis. The different members of this family can act in pro- (Bax, Bak) or anti-apoptotic (Bcl-xL, Bcl-2) ways. They form a very complex interaction network to manage the processes that induce the permeabilization of the mitochondrial outer membrane (MOM), which is the point of no return in the cell´s commitment to die2. Bax is a key member of this family believed to directly participate in this process. However, how the integration of Bcl-2 interactions regulates apoptosis remains poorly understood.
In healthy cells, Bax is a soluble monomeric protein constitutively shuttling between the cytosol and the mitochondrial surface. When a cell is stressed, Bax activates its killer instinct and accumulates at the MOM, where it inserts in symmetrical pairs 3. Once it is membrane-embedded, more Bax dimers are recruited leading to the formation of big aggregates that end up permeabilizing the membrane. This enables the release of cytochrome c and other death factors to the cytosol and irrevocably to cell death. Despite intense research, the mechanism that Bax follows to irreversibly damage the cell and the structures that the protein adopts during pore formation are still unsettled. In fact, the existence of these pores is under debate because nobody has managed to visualize them in the mitochondria of mammalian cells.
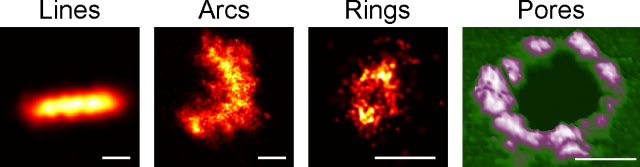
But seeing is believing, so in our latest work we have taken advantage of super-resolution microscopy as a tool to tackle the organization of Bax at the nanoscale during apoptosis 4. We used a combination of fluorescence, atomic force, and electron microscopy techniques to investigate Bax architecture in single cells. First, we corroborated that the spatial distribution of Bax evolves with time during the different stages of apoptosis progression. Fluorescence confocal microscopy showed that in healthy cells Bax localizes homogeneously in the cytosol but it moves to specific sites at mitochondria after an apoptotic insult (Fig 1). However, confocal microscopes do not have enough resolution to provide information about the structure or orientation of Bax assemblies on the membrane. Thanks to dual-color super-resolution microscopy we could observe for the first time the shape of these assemblies. Surprisingly, we discerned well-defined structures of Bax shaped as full rings, arcs and lines along mitochondria (Fig. 2). These characteristic “doughnuts”, “half-moons”, and “chains” are only present when Bax is active and the cell is dying, so they were not created by chance. Then, we checked if these Bax assemblies were really permeabilizing the mitochondrial membrane using atomic force microscopy. Strikingly, we observed that full rings and arcs of similar size (from 30 to 300 nm) are able to perforate membranes that mimic the composition of mitochondria (Fig 2). This confirms beyond doubt that the doughnuts and half-moons we detected are tightly related to Bax killer function. To further validate our findings, we also analyzed the mitochondria of apoptotic cells via electron microscopy. The cells exhibited disrupted mitochondrial surface, with holes in the same order of magnitude as the Bax structures identified in the super-resolution images.
In summary, this work proposes a new mechanism for Bax action and endorses the pore forming theory, providing an essential piece to assemble the intricate apoptotic puzzle.
There is still a long road ahead of us to fully understand how Bax and the other Bcl-2 proteins interact to kill the cells, but now that we have evidence of the existence of pores we are one step closer to completing the picture. When we finally untangle all the molecular details involved in apoptotic progression, we will be able to come up with ways to block or induce MOM permeabilization. This would open interesting possibilities for the development of therapies against cancer or neurological disorders.
References
- [Czabotar PE, Lessene G, Strasser A, Adams JM (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews Molecular cell biology 15: 49-63 ↩
- García-Sáez AJ (2012) The secrets of the Bcl-2 family. Cell Death Differ 19: 1733-1740 ↩
- Bleicken S, Jeschke G, Stegmueller C, Salvador-Gallego R, Garcia-Saez AJ, Bordignon E (2014) Structural model of active Bax at the membrane. Mol Cell 56: 496-505 ↩
- Salvador-Gallego R, Mund M, Cosentino K, Schneider J, Unsay J, Schraermeyer U, Engelhardt J, Ries J, Garcia-Saez AJ (2016) Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. The EMBO journal DOI: ↩
1 comment
[…] Aunque suene paradójico la destrucción de las células es un proceso tan importante como su reproducción; de hecho, el que no se autodestruyan es una de las características del cáncer. Raquel Salvador investiga los mecanismos de autodestrucción (apoptosis) de […]