Cut the stem off to fight breast cancer
Cut the stem off to fight breast cancer
Estrogens are steroid hormones affecting several organs including mammary glands. More precisely, these glands exert their action by means of estrogens through the estrogen receptor (ER), a transcription factor belonging to the family of nuclear receptors. So far, two types of ERs have been identified: alpha and beta. ER-beta is expressed in all mammary cells while ER-alpha is found in some particular cells (specifically, it is expressed in 20% of luminal cells, which form the lumen of the conducts and the mammary lobules). Experiments with mice have proved that if one of these genes is “removed”, this has an effect on the development of the gland. Thus, mice lacking the alpha receptor exhibit an anomalous breast epithelium, composed of rudimentary ducts. Mice lacking beta receptor show a normal morphology. In light of these results, ER-alpha must be the receptor intervening in the formation of the mammary gland.
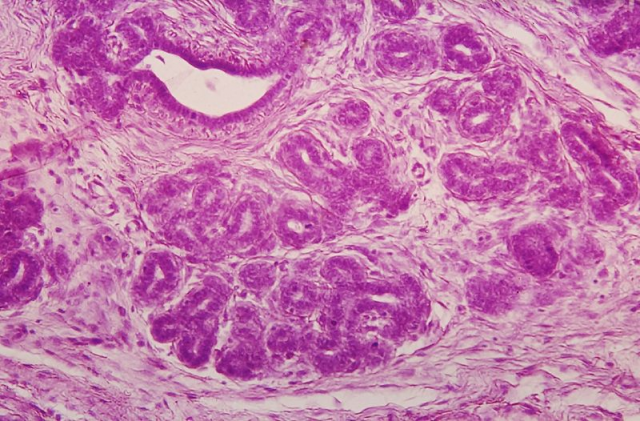
The mammary gland is a very dynamic organ, undergoing considerable changes over the lifetime of women. Mammary glands regenerate and grow throughout life due to the proliferation of epithelial cells. Therefore, this has suggested that there must be cells that are capable of dividing and differentiate into many types of specialized cells in the breast epithelium. These cells are commonly known as “stem cells”. Stem cells, which have become famous worldwide, are undifferentiated and can divide through mitosis and proliferate (self-renewal), giving rise to daughter cells that are genetically identical to the mother cells. On the other hand, they become tissue specialized cells by differentiation. Unlike stem cells, specialized cells are completely differentiated.
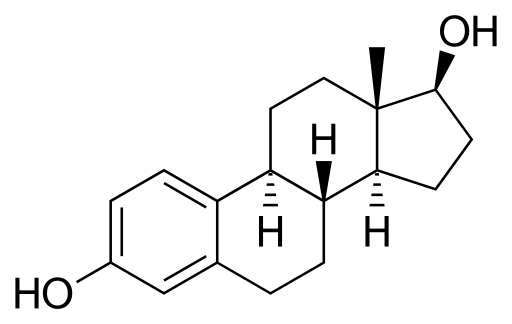
A paradoxical situation occurs in the mammary gland in relation to stem cells: estrogens reduce stem cell levels, but these cells do not possess ER. Estrogens exert this role indirectly through ER-containing cells, which are those cells surrounding stem cells. These ER-containing cells secrete proteins in response to estrogens and such proteins reach stem cells (without ER), inducing them to divide or differentiate.
Tumours are caused by an uncontrolled growth of cells inside the organism. Thereby, mammary tumours are generated from mammary cells that skip both outer and inner controls and start growing and dividing themselves, compromising the viability of the host organism. Knowing that estrogens and the ER are crucial for the functioning of the mammary gland, they may have something to say in the treatment of mammary tumours. In fact, the most extended classification of mammary tumours is based on the presence or absence of ER. Hence, tumours are divided into ER+ tumours (those tumours having ER alpha) and ER– (those lacing ER alpha). Differences at the clinical level are noteworthy. ER+ tumours represent 75% of tumours and have a better prognosis (chance of recovery) than ER– tumours, which are far more aggressive.
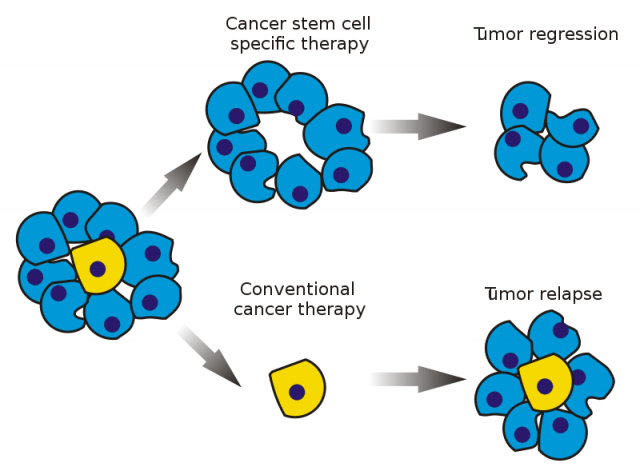
The antiestrogen tamoxifen is, so far, one of the most successful and widespread anti-ER+ mammary cancer treatments. It is an ER antagonist, that is, it binds to the receptor keeping it inactive and incapable of reacting to estrogenic signalling, resulting in cell death and elimination of the tumour mass. Nevertheless, we know now that mammary tumours are very heterogeneous, not only in relation to patients but also at the intratumoral level. It has been established that tumours also include stem cells (known as cancer stem cells), which may be responsible for tumour spreading and proliferation.
It is worth mentioning that those tumours with enhanced cancer stem cells present more aggressive characteristics and a more pessimistic prognosis. Resistance to current therapies comes along with an increase of tumour stem cells. In this context, for example, it has been proved that tumour stem cells do not express ER, which helps them to avoid the anti-proliferative effects of tamoxifen. For this reason, they may resist the treatment and generate another tumour, which represents a risk of relapse and a serious clinical problem. If we want to fight tumour stem cells we can either eliminate them or induce their differentiation. Retinoic acid, for instance, has been shown to stimulate this differentiation, provoking tumour cell breakdown.
In the near future, effective treatments will combine therapies against both tumour cells and tumour stem cells. In this sense, it is essential to study molecular mechanisms underlying the physiology of tumour stem cells. That way, we will be able to develop new treatments to fight this disease, and it this precisely what Dr. Vivanco’s group tries to do.
Dr. Vivanco’s lab considers tumour stem cells as therapeutic targets. Thanks to exhaustive research, the group found a gene 1 that was responsible for tamoxifen resistance in breast cancer cells. This gene, Sox2, which is also an embryonic stem cell marker, has as an increased expression in tamoxifen-resistant breast cancer cells and it is now considered a tamoxifen resistance biomarker.
In that work, it was shown that Sox2 silencing restores cell sensitivity to tamoxifen. Taken together, these results give hope to future development of effective treatments against breast cancer stem cells.
Other studies of the group have demonstrated that proliferation of breast cancer stem cells is associated to hypoxia, as this condition limits cancer stem cell differentiation through the expression of hypoxia-inducible factor 1-alpha or the inactivation of the prolyl hydroxilase 3.
On the whole, this new knowledge represents a breakthrough in breast cancer treatments, generating more effective combined therapies and, as an extension, they will hopefully open new pathways to understand stem cell physiology and their role in the proliferation of diverse tumours.
References
- M Pila et al (2014) Sox2 promotes tamoxifen resistance in breast cancer cells EMBO Molecular Medicine doi:
3 comments
[…] Read the complete article… […]
[…] Read the complete article… […]
[…] Check our article in Mapping Ignorance: Cut the stem off to fight breast cancer. […]