The skin I live in: the mineralocorticoid receptor
The mineralocorticoid receptor (MR) is an atypical nuclear receptor. In principle, it may seem a mere receptor with a specific ligand and an evident role: it is capable of detecting mineralocorticoids (like aldosterone), exerting an essential function controlling sodium and water reabsorption in the kidneys. In fact, knockout mice lacking MR die shortly after being born due to dehydration.
But things are not so easy in the biological world. The historical simplification “one gene, one function” is far from reality. With time, several experiments have shown the implication of MR in a broader range of processes and tissues, like, for instance, the skin. In this respect it has been proved that:
-
Certain drugs for treating hypertension that block MR as a mechanism of action have an effect on inflammatory and fibrotic skin diseases.
-
Mice overexpressing skin MR present epidermal atrophy and progressive alopecia.
Moreover, it must be considered that MR also recognises another ligand that, a priori, seems more likely to be the ligand of another nuclear receptor. We are talking about glucocorticoids (cortisol in humans), steroid hormones that are also recognized by the glucocorticoid receptor (GR).
Glucocorticoids are the base molecules to produce drugs that treat cutaneous diseases, although it has been proved that chronic treatments cause adverse effects (i. e. epidermal atrophy) that limit their therapeutic use. Is this pharmacologic action performed by GR or MR? Are there any differences? If so, could these be used to design effective treatments with no side effects?
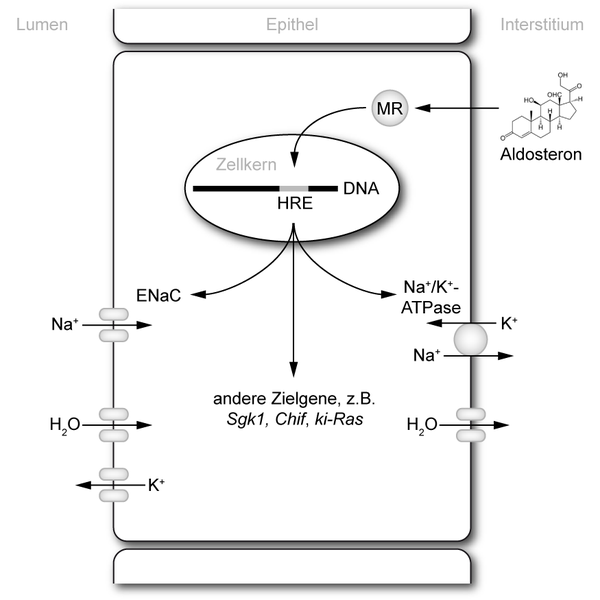
Dr. Pérez’s group tries to answer these questions. In one of the experimental approaches 1 they generated transgenic mice lacking MR only in epidermal keratinocytes, which are the predominant cells in the epidermis (90%). The rest of the cells presented MR, including the kidney, to keep the newborn mice from dying of dehydration. In fact, these mice were viable and fertile and showed minor skin alterations.
These transgenic specimens were created by crossing mice with a LoxP-flanked MR gene. These LoxP-flanked sequences are recognized and cut by the Cre enzyme, which is normally absent in mice. When these mice are crossed with Cre-containing mice, being the Cre gene controlled by the keratin 5 (a specific gene in skin cells) promoter, part of the offspring carry both genes. These mice presented MR in all their cells (where Cre is absent), except in those expressing keratin 5 (this is, skin cells), where Cre cuts LoxP sequences eliminating MR gene. Hence, these mice lack epidermal MR. The study of these mice leads to the identification of the role of this receptor in response to inflammatory stimuli and treatment with glucocorticoids.
By doing so, it was established that mice lacking MR in the skin present an increased reaction to irritant or inflammatory stimuli, as for example augmented skin damage and thickness. Those differences arose exclusively due to the absence of MR, because these mice already express GR in the normal way. Treatment with glucocorticoids produced epidermal thinning in those mice that were not modified (containing MR), which is partly attributed to decreased keratinocyte proliferation. In mice without skin MR did not affect so much to keratinocyte proliferation. For that reason, glucocorticoids exert some of their roles through MR being these effects unique, distinct from those functions characteristic of the glucocorticoid receptor.
In conclusion, the importance of this study lies in the generation of knockout mice for the mineralocorticoid receptor in skin cells, for this allows the investigation of MR specific role. According to the results, this receptor participates in a variety of epidermal processes. Furthermore, its roles are different in some way from the ones played by glucocorticoids. In this context, it is feasible to develop new drugs and improve glucocorticoid-based skin therapies. Using all this information it will be easier to achieve the formulation of new compounds with the same inflammatory properties but less secondary effects normally associated with these drugs, such as epidermal thinning.
References
- Julia Boix et al (2016) Epidermal Mineralocorticoid Receptor Plays Beneficial and Adverse Effects in Skin and Mediates Glucocorticoid Responses Journal of Investigative Dermatology doi: 10.1016/j.jid.2016.07.018 ↩
3 comments
[…] Gorputzaren homeostasirako ezinbestekoak dira azalaren funtzioak. Hartzaile nuklear bakar batek berebiziko papera du azalarentzat eta bizitzarentzat. NuRCaMein-ek The skin I live in: the mineralocorticoid receptor. […]
[…] Las funciones de la piel son vitales para la homeostasis del cuerpo. Un solo receptor nuclear tiene un papel importantísimo para la piel, y para la vida. NuRCaMein The skin I live in: the mineralocorticoid receptor. […]
[…] Check our latest article in Mapping Ignorance: BPA and pregnancy: diabetes for you and your kids. […]