Buckyball difluoride, a single-molecule crystal
Buckyball difluoride, a single-molecule crystal
Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex was synthesized in 1985 and called La@C60. The @ (at sign) in the name reflects the notion of a small molecule trapped inside a shell.
The chemistry of endohedral fullerenes is fascinating. Fullerenes interact with their encapsulated guest molecules either via noncovalent, dispersive and electrostatic forces or, because of fullerene’s high electron affinity, stabilize encapsulated cationic systems by forming covalent-type complexes with negatively charged cages and cationic endohedral guests (metals or metal clusters). Even some more exotic and unique bonding mechanisms between encapsulated species and fullerenes have been reported.
But all of the above assumes de buckyball (C60) is the electron-hungry partner and that the encapsulated molecule is an electron donor or, directly, a cation. But, could it be the other way around? Could we conceive of a system where the fullerene is an electron donor?
In spite of extensive studies conducted on diverse families of endohedral fullerenes, no system with an endohedral anion and a positively charged fullerene has been detected or proposed. Until now.
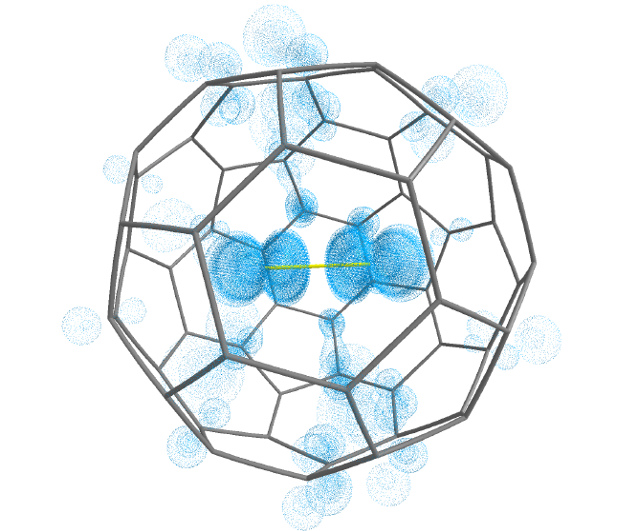
A team of researchers, incluiding DIPC’s Gernot Frenking, has studied 1 a series of halogen molecules encapsulated in C60, X2@C60 (X=F, Cl, Br, I). As a result, the researchers demonstrate that F2@C60 is an unprecedented molecular system with negatively charged F2– inside a positively charged C60+, which can be a viable synthetic target through fullerene surgery.
Energy decomposition analysis, in conjunction with natural orbitals for chemical valence computations were carried out by the team for the singlet and triplet states of F2@C60 using 2F2– and C60+as interacting fragments. An additional calculation was performed for the 3,1F2@C60 using neutral F2 and C60 as fragments. It turned out that the charged fragments 2F2– and C60+provide a better description for the bonding situation in F2@C60 than using the neutral fragments.
In other words, there is essentially no covalent bonding between 2F2– and C60+. We are before a unique bonding situation in F2@C60 in which an electron is transferred from the C60 cage, of all things an electron deficient system that normally accommodates up to 6 electrons to fill its shell, F2, thus forming the F2–@C60+ system. This charge separation between C60 and F2 takes place without covalent bond formation, a situation which is otherwise only found in perfect ionic crystals. Thus, F2@C60 could be termed a single-molecule crystal compound.
As a side effect these results may shed a new light on the oxidation process of organic compounds by fluorine. It is generally believed that the fluorination reaction starts by the homolytic cleavage of F2 bond. In F2@C60, however, F2 efficiently abstracts one electron from electron deficient C60. This can be the case for less electron deficient compounds in the initiation step of the fluorination reaction.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Cina Foroutan-Nejad, Michal Straka, Israel Fern#ndez, and Gernot Frenking (2018) Buckyball Difluoride F2–@C60+ — A Single-Molecule Crystal Angewandte Chemie International Edition doi: 10.1002/anie.201809699 ↩