Tracking the tautomerization of a single molecule in space and time
Tracking the tautomerization of a single molecule in space and time
There are chemical compounds, called isomers, that have the same molecular formulae but different molecular structures or different arrangements of atoms in space. In constitutional isomerism the molecules have different molecular structures: i.e., they may be different types of compound, or they may simply differ in the position of the functional group in the molecule. Constitutional isomers generally have different physical and chemical properties. In some cases, these isomers are in equilibrium and are known as tautomers.
Tautomerization, the interconversion between two tautomers, is ubiquitous in nature. It also plays a major role in chemistry and is considered an ideal switch function for emerging molecular-scale devices. It seems important, then, to know what variables, at atomic scale, affect this process.
Take the case of porphyrins. Porphyrins are organic pigments characterized by the possesion of a cyclic group of four linked nitrogen-containing rings called porphin (Figure 1), the nitrogen atoms of which are often coordinated to metal ions. Porphyrins differ in the nature of their side-chain groups. They include the chlorophylls, which contain magnesium; and haem which contains iron and forms the prosthetic group of haemoglobin, myoglobin and the cytochromes. In porphyrins, tautomerization means the concerted or sequential hopping of the two inner hydrogen atoms between equivalent nitrogen sites of the molecular cavity. The same happens in phtalocyanine, a synthetic compound with a structure similar to porphyrins (Figure 2).

The electronic and vibronic changes that result from this NH tautomerization, as well as details of the switching mechanism, have been extensively studied with optical spectroscopies, even with single-molecule sensitivity. On the other hand, the influence of atomic-scale variations of the molecular environment and submolecular spatial resolution of the tautomerization could only be investigated using scanning probe microscopes, at the expense of the detailed information provided by optical spectroscopies.
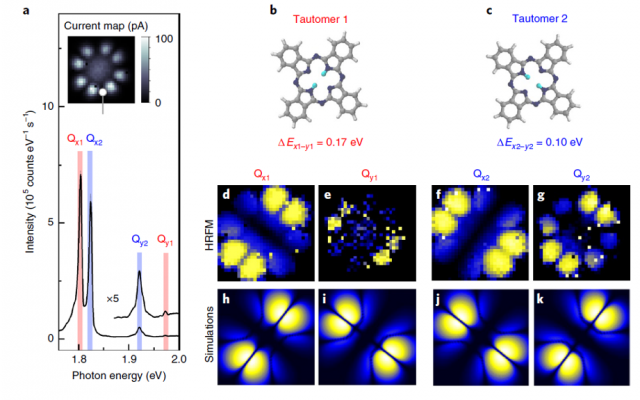
Now, a team of researchers combines 1 these two approaches, scanning tunnelling microscopy (STM) and fluorescence spectroscopy, to study the tautomerization within a prototypical single-molecule switch, individual free-base phthalocyanine molecules deposited on a NaCl-covered Ag(111) single-crystal surface.
STM-induced fluorescence spectra exhibit duplicate features that the scientists assign to the emission of the two molecular tautomers. This interpretation is supported by comparing hyper-resolved fluorescence maps of the different spectral contributions with simulations that account for the interaction between molecular excitons and picocavity plasmons.
The researchers are able to identify the orientation of the molecular optical dipoles, determine the vibronic fingerprint of the tautomers and probe the influence of minute changes in their atomic-scale environment. The tautomerization events are monitored via time-correlated fluorescence measurements, what makes possible to associate the proton dynamics to a switching two-level system.
Finally, optical spectra acquired with the tip located at a nanometre-scale distance from the molecule show that the tautomerization reaction occurs even when the tunnelling current does not pass through the molecule. Together with other observations, this remote excitation indicates that the excited state of the molecule is involved in the tautomerization reaction path. By developing a simulation method that accounts for both the excitation and deexcitation probabilities in an STM-F experiment, in which molecular switches are shown to modulate only the current traversing a nanoscale circuit, the scientists demonstrate that a molecular optoelectronic element can also be used to activate an optical signal.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may be copied verbatim or almost verbatim from the referenced research paper.
References
- Benjamin Doppagne, Tomáš Neuman, Ruben Soria-Martinez, Luis E. Parra López, Hervé Bulou, Michelangelo Romeo, Stéphane Berciaud, Fabrice Scheurer, Javier Aizpurua and Guillaume Schull (2020) Single-molecule tautomerization tracking through space- and time-resolved fluorescence spectroscopy Nature Nanotechnology doi: 10.1038/s41565-019-0620-x ↩