Beyond lithium: magnesium rechargeable batteries
Beyond lithium: magnesium rechargeable batteries
Lithium-ion batteries have remained unrivalled in terms of overall performance for several applications, as evidenced by their widespread use in everything from portable electronics to cellular base stations. However, they suffer from some important disadvantages that are difficult to ignore. For one, lithium is rather expensive, and the fact that it is being mined at an extreme pace does not help. Moreover, the energy density of lithium-ion batteries is not enough to grant ample autonomy to electric vehicles and heavy machinery. These concerns, coupled with the fact that the batteries are highly unsafe when punctured or at high temperatures, have caused scientists to look for alternative technologies. Among the various elements being tested as efficient energy carriers for rechargeable batteries, magnesium (Mg) is a promising candidate. Apart from its safety and abundance, Mg has the potential to realize higher battery capacities. However, some problems need to be solved first. These include the low voltage window that Mg ions provide, as well as the unreliable cycling performance observed in Mg battery materials.
To tackle these issues, one approach is to look for new cathode materials for Mg batteries. Now, a team of scientists has shared their results 1 after searching for ways to improve the performance of cathode materials based on the MgV (V: vanadium) system.
The researchers focused on the Mg1.33V1.67O4 system but substituted some amount of vanadium with manganese (Mn), obtaining materials with the formula Mg1.33V1.67−xMnxO4, where x goes from 0.1 to 0.4. While this system offered high theoretical capacity, more details about its structure, cyclability, and cathode performance needed to be analysed to understand its practical utility. Accordingly, the researchers characterized the synthesized cathode materials using a wide variety of standard techniques.
First, they studied the composition, crystal structure, electron distribution, and particle morphologies of Mg1.33V1.67−xMnxO4 compounds using X-ray diffraction and absorption, as well as transmission electron microscopy. The analyses showed that Mg1.33V1.67−xMnxO4 has a spinel structure with a remarkably uniform composition.
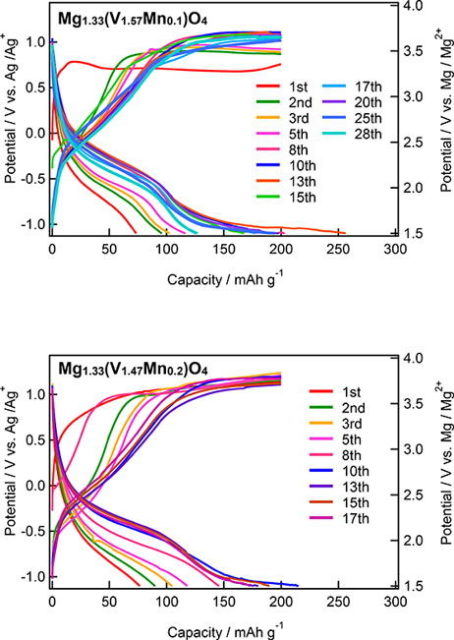
Next, the researchers conducted a series of electrochemical measurements to evaluate the battery performance of Mg1.33V1.67−xMnxO4, using different electrolytes and testing the resulting charge/discharge properties at various temperatures.
The team observed a high discharge capacity for these cathode materials—especially Mg1.33V1.57Mn0.1O4—but it also varied significantly depending on the cycle number. To understand why, they analyzed the local structure near the vanadium atoms in the material. The particularly stable crystal structure along with a large amount of charge compensation by vanadium leads to the superior charge–discharge properties observed for Mg1.33V1.57Mn0.1O4. Taken together, the results indicate that Mg1.33V1.57Mn0.1O4 could be a good candidate cathode material for magnesium rechargeable batteries.
Future research and development may show that magnesium batteries could surpass lithium-ion batteries, thanks to the former’s higher energy density. Substituted MgV systems could eventually lead to the much awaited next-generation batteries.
References
- Idemoto, Y. et al (2022) Electrochemical properties and crystal and electronic structure changes during charge/discharge of spinel type cathode-materials Mg1.33V1.67−xMnxO4 for magnesium secondary batteries Journal of Electroanalytical Chemistry doi: 10.1016/j.jelechem.2022.117064 ↩