How lithium ions move in substituted ceramic solid electrolytes

From electric vehicles to storing wind and solar energy produced during off-peak hours, rechargeable lithium-ion (Li-ion) batteries are the best option that we currently have tu support our technological future. But if we want them to be truly environmentally-friendly and safe devices, they do need some changes.
The Li-ion battery is a rechargeable cell. Typically, the anode is carbon and the cathode is a metal oxide like cobalt (IV) oxide. The electrolyte is a lithium salt such as the borate or chlorate in an organic solvent. The action of the cell depends on the movement of Li ions – hence, the name –between anode and cathode with oxidation of the cobalt ions during charging and reduction during discharge. This design allows Li-ion batteries to be light and have a low self-discharge rate, although the capacity does deteriorate with age.
The environmental and safety concerns in a Li-ion battery, for most applications, concentrate on the toxic and flammable liquid organic solvent. An efficient inorganic solid alternative would be the solution. But it is not that easy, because solids are tricky.
There are many good Li-ion solid electrolyte candidates. Among them, Li7La3Zr2O12 (LLZO) garnet is a strong contender in terms of good Li-ion conductivity, wide electrochemical operation window, and formation of stable interfaces with many cathode materials. But there is a catch.
LLZO garnets can crystallize in different polymorphs, a poorly Li-ion conductive tetragonal structure and a much more conductive cubic one are the main ones, plus an additional acentric cubic. The cubic structure is unstable at room temperature, but it can be stabilized by supervalent substitution at the Li, La, or Zr crystallographic sites. In particular, substitution of Li by Al and Ga ions has extensively been studied, with most efforts focused on elucidating the site preferences of Al and Ga and their effect on Li-ion conduction in LLZO garnets.
The point is that, despite having the same formal charge, Ga substitution is found to lead to higher room temperature conductivities than Al substitution, but nobody really knows why.
Now, a team of researchers from CIC EnergiGUNE and BCAM has investigated this phenomenon 1 in order to gain an atomic-level insight into the different role the similar substitutional metal ions (Al, Ga) have in the bulk Li-ion mobility of LLZO ceramic electrolytes.
The researchers have applied force-field-based molecular dynamics (MD) simulations to a range of model systems and temperatures. In addition to conventional MD simulations, and in order to effectively account for exhaustive sampling at low temperatures, a Generalized Shadow Hybrid Monte Carlo (GSHMC) approach has been applied for the first time to study single and dual substituted LLZO systems.
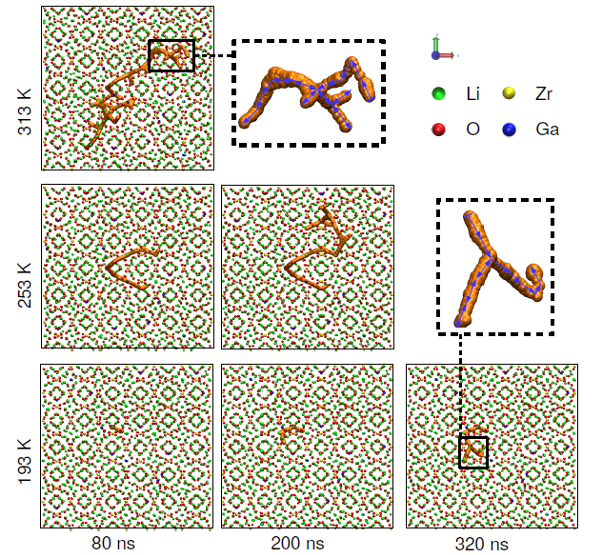
different temperatures. From top to bottom the temperature decreases, whereas from left to
right the simulation time increases. Li, Zr, O and Ga ions are depicted in green, yellow, red
and blue spheres, respectively (La ions are not shown for simplicity).
The calculations show that increasing Ga content produces a subtle expansion of the unit cell volume at all investigated temperatures, from -80ºC (193 K), the first time that such a low temperature region is explored in force-field simulations of garnet materials, to 120ºC (393 K). This implies that while Ga3+ ions tend to allow limited Li+ diffusion within their immediate surroundings, the less repulsive interactions associated with Al3+ ions lead to a complete blockage of neighboring Li+ diffusion paths. This effect is magnified at lower temperatures, and explains the higher conductivities observed for Ga-substituted systems.
These results pave the way for rationalizing design strategies based on the use of atoms of different valence for enhancing ionic transport in these materials, and bring closer the next generation of Li-ion batteries.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Fabián A. García Daza, Mauricio R. Bonilla, Anna Llordés, Javier Carrasco, and Elena Akhmatskaya (2019) Atomistic Insight into Ion Transport and Conductivity in Ga/Al-Substituted Li7La3Zr2O12 Solid Electrolytes ACS Applied Materials & Interfaces doi: 10.1021/acsami.8b17217 ↩
2 comments
[…] Antzeman dezakegunarengatik gerta daiteke etorkizunean bateriak izatea hainbat konturen oinarri. Bateria hauek egun diren baino seguruagoak izan beharko dute eta horretarako, erabiltzen diren elektrolito organikoak ezabatu beharko dira toxikoak eta sukoiak baitira. Egokiena elektrolito solidoak erabiltzea litzateke baina horretarako […]
[…] Por lo que podemos vislumbrar, el futuro es probable que se base en baterías. Estas tendrán que ser más seguras de lo que son hoy día y para ello habrá que eliminar los electrolitos orgánicos líquidos que emplean, ya […]