SARS-CoV-2 infect immune cells of the central nervous system
SARS-CoV-2 infect immune cells of the central nervous system
SARS-CoV-2
Author: José R. Pineda got his Ph.D. from University of Barcelona in 2006. Since 2007 he has worked for Institut Curie and The French Alternative Energies and Atomic Energy Commission. Currently he is a researcher of the UPV/EHU. He investigates the role of stem cells in physiologic and pathologic conditions.
With 676.609.955 cases registered worldwide and a toll of 6.881.955 total deaths, SARS-CoV-2 virus has shattered both global health and economy worldwide. The Omicron variant emerged in October 2021 increasing its transmissibility and immune evasion. Despite the end of public health, emergency declaration does not mean COVID-19 is gone. Previous studies determined that SARS-CoV-2 could reach brain parenchyma and even reduce grey matter. The brain is composed of various cell types that can be classified as cell lineages: all the neurons belongs to the neuronal cell lineage and responsible for synaptic transmission (gray matter is composed of neuronal bodies). Then, we have macroglia in which we differentiate astrocytes and oligodendrocytes. Astrocytes belongs to astroglial cell lineages and are responsible to feed, support, and fine-tune synaptic transmission of the neurons. Oligodendrocytes belongs to oligodendroglial lineage and are responsible for myelination and remyelination of neuronal axons. Finally, we have microglia, composed of myeloid cell lineages (originated from yolk sac-primitive macrophages) that is the first and main form of active immune defense surveilling for pathogens or tissue damage in the central nervous system.
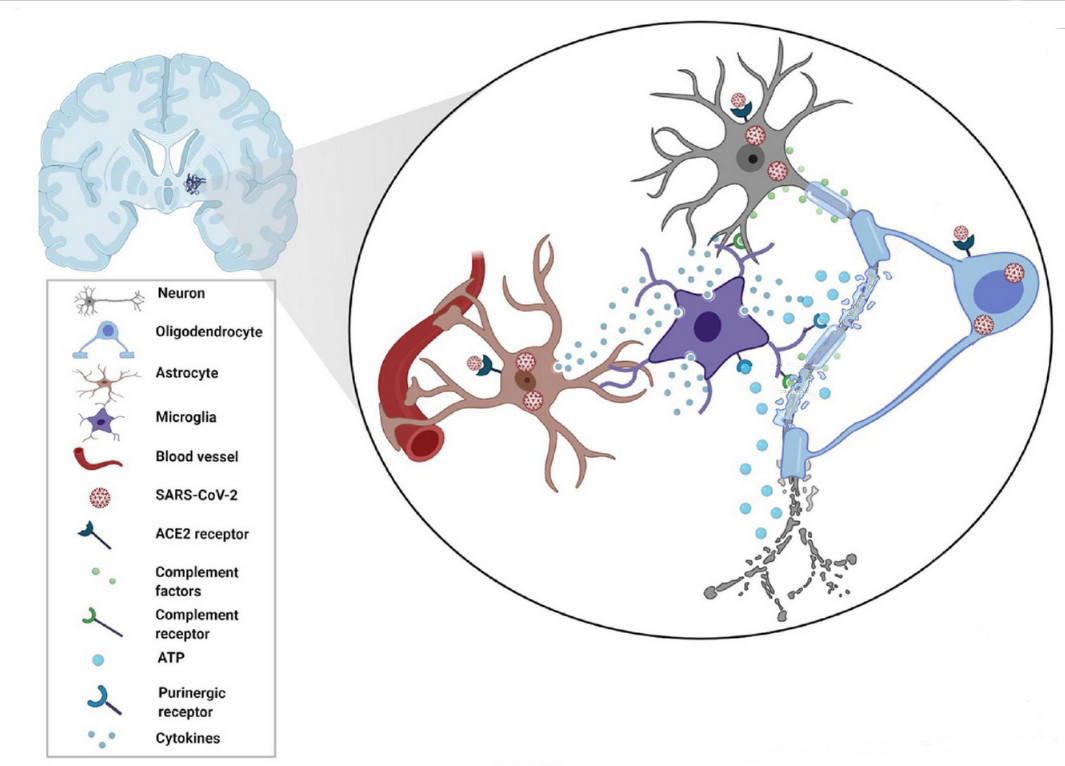
The article published by Yoshitaka Kase and collaborators [4] highlighted a new unexpected finding, SARS-CoV-2 variants also changed the cellular preference for infections, with the original strain of SARS-CoV-2, the Delta variant, and the Omicron variant able to infect very efficiently the microglial cells. Kase and collaborators used the differentiation of human induced pluripotent stem cells (hiPSCs) as a strategy to obtain the different type of cells, cortical neurons, astrocytes and microglia to be infected. Moreover, in a second experimental phase, they created brain organoids to corroborate the results.
Due to legal restrictions to directly handling SARS-CoV-2, the authors modified lentiviral particles, removing the G protein of the vesicular stomatitis virus (VSV-G) and expressing the S protein (key protein of the spike to become infective). The first and unexpected result was that SARS-CoV-2 hardly infected cortical neurons. They used each variant of S protein for the original strand, the Delta variant (B.1.617.2 strain) and the Omicron variant (B.1.1.529 strain). Moreover, they incorporated a fluorescent protein reporter EGFP following the EF1a promoter as an indicator of successful infection. The researchers performed the infections during 24h and changed culture media to remove the virus, waiting for 48h to allow the induction (or not) of EGFP expression and then fixed the cells. Next, they performed immunofluorescence against EGFP and a neuronal marker -III-tubulin and did not find infection of neurons.
They completed the immunofluorescence against EGFP and the glial fibrillary acidic protein (GFAP, an intermediate filament-III protein uniquely found in astrocytes in the CNS) and again did not find infection of astrocytes. Next, they moved to cerebral organoids cultured for 30 days. Due to the size of the cellular mass, at the end of the experiment they fixed the culture and sectioned to do immunofluorescence without observing any EGFP positive staining. Finally, they tested SARS-CoV-2 infection on microglial cells generated from iPSCs checking the double colocalization of Iba-1 microglial marker and EGFP staining for both Delta and Omicron variants. From previous literature they found that cells expressing dipeptidyl peptidase 4 (DPP4, a protein associated to immune cell regulation and glucose metabolism) and the CD147 receptor (also expressed in platelets and megakaryocytes involved in its hyperactivation and thrombosis) were targets recognized by the coronavirus to infect the cells. They found that DPP4 was significantly overexpressed in microglial cells, concluding that DPP4 is an essential factor in whether SARS-CoV-2 infection is viable in cells of the CNS.
Their findings are of remarkable importance due to the role of microglia is not exclusively restricted to respond to infections but also has a close interaction with neurons and astrocytes promoting synaptic pruning and formation and maintaining CNS homeostasis. Thus, SARS-CoV-2 infection has been found to promote microglial synapse elimination in human brain organoids and produce a proinflammatory microglial activation inducing apoptotic cell death. Because the virus is cytocidal, one of the expected effects may be the reduction of microglial cell populations. Previous studies focused on the characterization of microglial depletion either pharmacologically or using conditional mutants to remove microglia have shown that its absence results in cognitive and learning deficits in rodents during development, but this effect is less pronounced in adults. However, evidence suggests that microglia play a role in cognition and learning in adulthood and, at a cellular level, may modulate adult neurogenesis and potential neurodegeneration.
In conclusion, the work of Kase and collaborators found that the spikes (molecular machinery) of SARS-CoV-2 can infect microglia since its emergence and was a regained function with the most infective forms Delta variant (B.1.617.2 strain) and the Omicron variant (B.1.1.529 strain). These findings shed new light to understand the many COVID-19-related CNS disorders that were reported at the beginning of the pandemic and should prepare us for the long-term possible consequences during ageing.
References:
[1] Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Inf Dis. 20(5):533-534. doi: 10.1016/S1473-3099(20)30120-1
[2] Jenny Meinhardt, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature Neuroscience. 2020 Nov 30. doi: 10.1038/s41593-020-00758-5.
[3] Douaud G, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022 Mar 7. doi: 10.1038/s41586-022-04569-5.
[4] Yoshitaka Kase, Iki Sonn, Maraku Goto, Rei Murakami, Tsukika Sato, Hideyuki Okano. The original strain of SARS-CoV-2, the Delta variant, and the Omicron variant infect microglia efficiently, in contrast to their inability to infect neurons: Analysis using 2D and 3D cultures. Exp Neurol. 2023 May;363:114379. doi: 10.1016/j.expneurol.2023.114379.
[5] Veronique E Miron, Tanja Kuhlmann, Jack P Antel. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta. 2011 Feb;1812(2):184-93. doi: 10.1016/j.bbadis.2010.09.010.
[6] W Y Chan 1 , S Kohsaka, P Rezaie. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007 Feb;53(2):344-54. doi: 10.1016/j.brainresrev.2006.11.002.
[7] Filiano AJ, Gadani SP, Kipnis J. Interactions of innate and adaptive immunity in brain development and function. Brain Research. 2015, 1617: 18–27. doi:10.1016/j.brainres.2014.07.050
[8] Andoh M., Ikegaya Y., Koyama R. Synaptic Pruning by Microglia in Epilepsy. J. Clin. Med. 2019;8:2170. doi: 10.3390/jcm8122170.
[9] DePaula-Silva A.B., Gorbea C., Doty D.J., Libbey J.E., Sanchez J.M.S., Hanak T.J., Cazalla D., Fujinami R.S. Differential transcriptional profiles identify microglial- and macrophage-specific gene markers expressed during virus-induced neuroinflammation. J. Neuroinflamm. 2019;16:152. doi: 10.1186/s12974-019-1545-x.
[10] Samudyata, Oliveira, A.O., Malwade, S. et al. SARS-CoV-2 promotes microglial synapse elimination in human brain organoids. Mol Psychiatry 27, 3939–3950 (2022). https://doi.org/10.1038/s41380-022-01786-2
[11] Jeong GU, Lyu J, Kim KD, Chung YC, Yoon GY, Lee S, Hwang I, Shin WH, Ko J, Lee JY, Kwon YC. SARS-CoV-2 Infection of Microglia Elicits Proinflammatory Activation and Apoptotic Cell Death. Microbiol Spectr. 2022 Jun 29;10(3):e0109122. doi: 10.1128/spectrum.01091-22. Epub 2022 May 5. PMID: 35510852; PMCID: PMC9241873.
[12] Green KN, Crapser JD, Hohsfield LA. To kill a microglia: a case for CSF1R inhibitors. Trends Immunol. 2020;41:771–784.
[13] David Graykowski and Eiron Cudaback. Don’t know what you got till it’s gone: microglial depletion and neurodegeneration. Neural Regen Res. 2021 Oct; 16(10): 1921–1927. doi: 10.4103/1673-5374.308078.
[14] Awogbindin IO, Ben-Azu B, Olusola BA, Akinluyi ET, Adeniyi PA, Di Paolo T and Tremblay M-È (2021) Microglial Implications in SARS-CoV-2 Infection and COVID-19: Lessons From Viral RNA Neurotropism and Possible Relevance to Parkinson’s Disease. Front. Cell. Neurosci. 15:670298. doi: 10.3389/fncel.2021.670298