X-ray fluorescence imaging, a pivotal tool in modern biological research
X-ray fluorescence imaging, a pivotal tool in modern biological research
X-ray fluorescence imaging (XFI) is a powerful technique that enables scientists to visualize and map the distribution of elements within various samples, including biological tissues. By detecting the unique fluorescent signals emitted by elements when they are excited by X-rays, XFI provides detailed information about the elemental composition and spatial distribution in a non-destructive manner. This technology has been instrumental in advancing our understanding of complex biological processes and disease mechanisms.
Principles of X-ray Fluorescence Imaging
At its core, XFI relies on the interaction between X-rays and matter. When a sample is exposed to high-energy X-rays, the atoms within the sample absorb this energy, causing inner-shell electrons to be ejected. This creates vacancies that are subsequently filled by electrons from higher energy levels, resulting in the emission of characteristic fluorescent X-rays specific to each element. By detecting these emitted X-rays, XFI can identify and quantify the elements present in the sample.
One of the significant advantages of XFI is its ability to detect trace elements with high sensitivity and spatial resolution. This makes it particularly valuable in biological research, where understanding the distribution of elements like iron, zinc, and copper is crucial for elucidating their roles in physiological and pathological processes.
Applications in Biological Research
XFI has been widely adopted in various fields of biological research due to its versatility and precision. For instance, it has been used to study metal ion homeostasis in cells, investigate the distribution of therapeutic drugs within tissues, and analyze the elemental composition of single cells. The non-destructive nature of XFI allows for the preservation of samples, enabling further analyses if necessary.
Case Study 1: Investigating the Protein Corona around Nanoparticles
Nanoparticles are increasingly being explored for biomedical applications, including drug delivery and imaging. When introduced into biological fluids, nanoparticles quickly adsorb proteins onto their surface, forming a “protein corona.” This corona influences the nanoparticles’ biological identity, affecting their interactions with cells and their overall therapeutic efficacy.
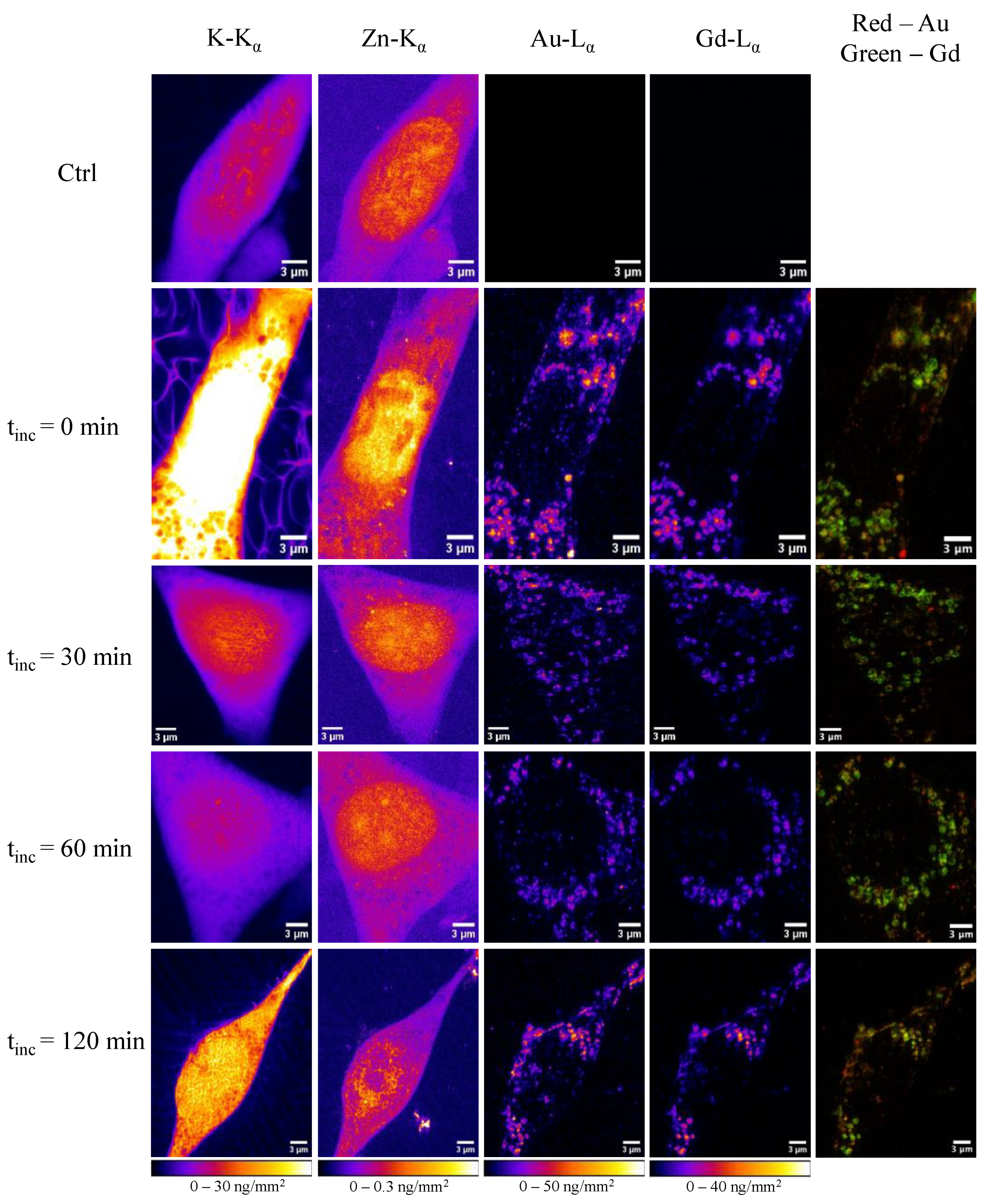
In a study 1, researchers utilized nanofocused XFI to investigate how the protein corona evolves within cells. By labelling the proteins in the corona with specific elements, they were able to track the intracellular distribution of both the nanoparticles and the associated proteins. This approach provided insights into how nanoparticles are processed within cells and how the protein corona influences their intracellular fate.
The findings from this study have significant implications for the design of nanoparticle-based therapeutics. Understanding the interactions between nanoparticles and the protein corona can lead to the development of more effective and targeted drug delivery systems, minimizing potential side effects and improving patient outcomes.
Case Study 2: Exploring the Intracellular Distribution of Selenium Compounds Delivered by Biodegradable Polyelectrolyte Capsules
Selenium compounds have garnered attention for their potential anticancer properties. However, delivering these compounds effectively to cancer cells while minimizing toxicity to healthy tissues remains a significant challenge. In a study 2, researchers investigated the use of biodegradable polyelectrolyte capsules as carriers for selenium compounds.
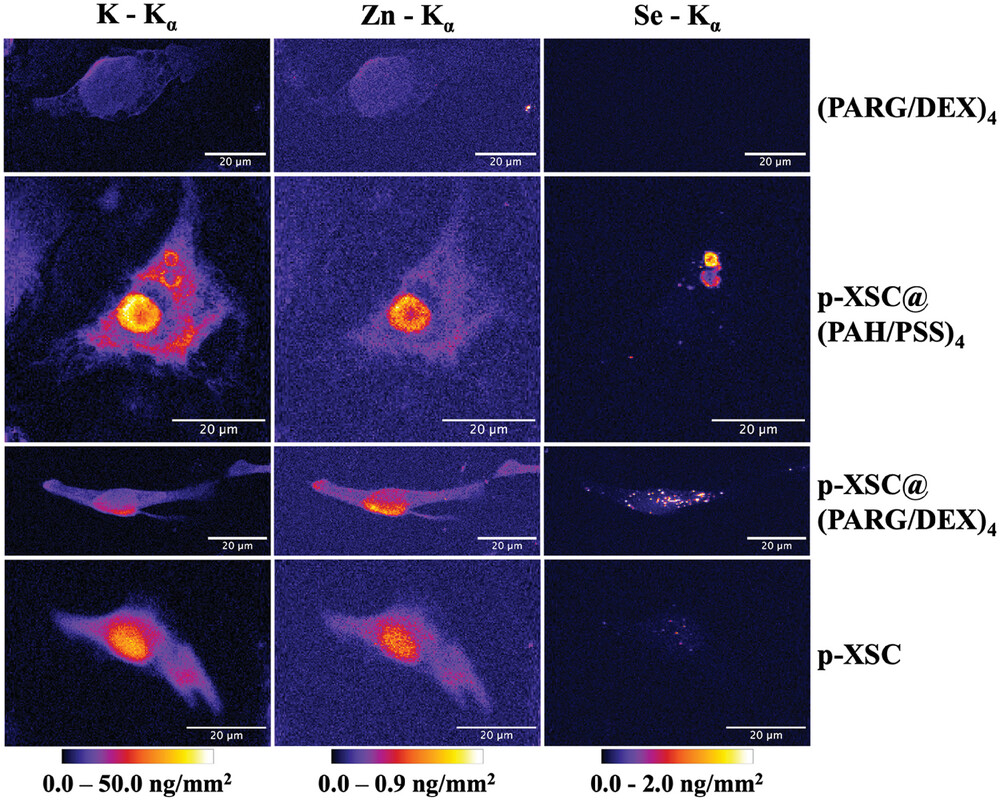
The study employed XFI to track the distribution of selenium within cancer cells after delivery by these capsules. The imaging revealed that the selenium compounds were successfully delivered into the cells and localized in specific intracellular compartments. This precise delivery is crucial for maximizing the therapeutic effects of selenium while minimizing potential side effects.
The insights gained from this research highlight the potential of using biodegradable carriers to enhance the delivery and efficacy of anticancer agents. By ensuring that therapeutic compounds reach their intended targets within cells, such strategies can improve treatment outcomes and reduce adverse effects.
Future Perspectives
As XFI technology continues to evolve, its applications in biological and medical research are expected to expand further. The development of more compact and cost-effective XFI systems will likely lead to their integration into clinical settings, aiding in disease diagnosis and the monitoring of therapeutic interventions.
Moreover, combining XFI with other imaging modalities, such as magnetic resonance imaging (MRI) or positron emission tomography (PET), could provide complementary information, offering a more comprehensive view of biological systems. This multimodal approach has the potential to revolutionize our understanding of complex diseases and contribute to the development of personalized medicine strategies.
X-ray fluorescence imaging stands as a pivotal tool in modern biological research, offering unparalleled insights into the elemental composition of biological samples. Its ability to provide high-resolution, non-destructive imaging makes it invaluable for studying intricate biological processes and disease mechanisms. The advancements highlighted in the discussed studies underscore the dynamic nature of XFI technology and its promising future in both research and clinical applications.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Skiba, M., Guedes, G., Karpov, D., Feliu, N., L. Cortajarena, A., Parak, W. J., & Sanchez-Cano, C. (2024). Probing the Cellular Fate of the Protein Corona around Nanoparticles with Nanofocused X-ray Fluorescence Imaging International Journal of Molecular Sciences doi: 10.3390/ijms25010528 ↩
- M. Skiba, R. R. Reszegi, Y. Huang, S. Roy, J. Han, D. Brückner, C. Sanchez-Cano, Y. Zhao, M. Hassan, N. Feliu, G. Falkenberg, W. J. Parak (2024) Exploring the Intracellular Distribution of Se Compounds Delivered by Biodegradable Polyelectrolyte Capsules Using X-Ray Fluorescence Imaging. Adv. Funct. Mater. doi: 10.1002/adfm.202408539 ↩