Immune signals affect neural circuits involved in schizophrenia
Immune signals affect neural circuits involved in schizophrenia
Author: José R. Pineda got his Ph.D. from University of Barcelona in 2006. Since 2007 he has worked for Institut Curie and The French Alternative Energies and Atomic Energy Commission. Currently he is a researcher of the UPV/EHU. He investigates the role of stem cells in physiologic and pathologic conditions.
Schizophrenia is a chronic and severe mental disorder that affects how a person thinks, feels, and behaves. It is characterized by episodes of psychosis, which can include hallucinations (seeing or hearing things that are not there), delusions (false beliefs), disorganized thinking with impaired emotional expression. The symptoms of schizophrenia can be classified as “positive” (hallucinations, delusions, disorganized speech or abnormal motor), “negative” (lack of motivation, social withdrawal, anhedonia) or “cognitive” in which individuals had impaired working memory and difficulty with decision-making/problem-solving skills. Other common problems can be to have troubles to focus or pay attention in some tasks. Indeed, the symptomatology is very heterogeneous and the causative factors underlying the disease are very complex 1 2. Despite the causes and risk factors are unknown, genetic and environmental and prenatal factors has been proposed to be involved in its etiology. However, recent evidences adds new actor in this complex relationship such as TNF-α or IL-6 inflammatory cytokines correlating with “negative” and “cognitive” symptoms in schizophrenia 34. Interestingly, a previous work published some years ago reported that some brain regions were invaded by lymphocytes (a type of white blood cell) or had aberrant microglia (the brain “special” immune system) distribution. This is interesting due to these are major actors to control these cytokine communications and both has been found in patients with prevalence of “negative” symptomatology 5.
The Ikaros family
A molecular bridge between the neural and immune system are the “Ikaros” family of transcription factors (TFs). They have been described to be crucial for the normal development of immune cells 6 and for the generation of late-born striatal neurons 7. In a recent work, Ballasch and collaborators found that altered mRNA levels and function of IKZF1 and IKZF2 genes fromIkaros family could affect the communication between the immune and central nervous systems during the progression of schizophrenia 8. To demonstrate that, they analyzed the levels of these two transcription factors in human samples of brain and circulating immune cells, and generated mutant mice KO of both Ikzf1, Ikzf2. Finally, to fully corroborate the “cause-consequence” of these changes they elegantly transferred the secretome of human circulating immune cells infused into the brains of living mice and evaluated their schizophrenia-like phenotypes.
A novel molecular mechanism involved in schizophrenia
First of all the authors decided to analyze in post-mortem human brain samples if there were significant changes of the activation of both IKZF1 and IKZF2 genes. To do that they analyzed both mRNA levels (using RT-qPCR) and their proteins (using the western blot technique) in different brain regions affected in the pathophysiology of schizophrenia: the hippocampus (this structure is involved in mood and spatial memory), the dorsolateral prefrontal cortex (involved in executive functions, such as working memory, cognitive flexibility, planning, inhibition, and abstract reasoning) and the putamen (involved in the regulation of movements at various stages such as in preparation and execution; and to influence various types of learning). They compared samples from patients with schizophrenia and control patients but no significant differences in any region were found. Because previous data linked the absence of this genes in brain-related deficiencies, and knowing that both IKZF1 and IKZF2 genes are more overexpressed in lymphocytes (white blood cells) than in neural tissues, they isolated peripheral blood mononuclear cells (~ 70–90% lymphocytes). They found that both genes were significantly downregulated in the samples from schizophrenia but only IKZF1 protein was downregulated. Because there are several type of lymphocytes, they sought to analyze each subpopulation finding a specific downregulation of both mRNA IKZF1 & IKZF2 in the CD4+ lymphocytes (also known as “T helper cells” that release cytokines to coordinate the response of the full adaptative immune system). Because human patients suffering schizophrenia usually are medicated, to exclude the possibility that medication could influence their levels, they decided to medicate mice in which they isolated and measured the mRNA Ikzf1 & Ikzf2of these cells. No changes were found between medicated and non-medicated cohorts.
Next step was to mimic the double Ikzf1 and Ikzf2 downregulation in mice. Double “knock-out” (“KO”) mutant mice were generated. Surprisingly they found that these animals developed schizophrenia like disturbances with all three symptom dimensions: positive (increased basal locomotion/agitation in the open field paradigm with increased senitivity to D-Amphetamine), negative (alteration in sociability), and cognitive (using the “novel object recognition test” the animals were uncapable to recognize the new object). All these observed anomalies could underlie in neuronal alterations. To corroborate that, neuronal connections were analyzed. Neurons connect making synapses, and the synapse is done when an axon contacts with a dendritic spine. Therefore, they analyzed the spine density of the neurons of the hippocampus, prefrontal cortex and putamen. They found a significant reduction of dendritic spine density in the putamen, and, interestingly in the hippocampus of mice males (but no females). However, they did not found any differences in the prefrontal cortex. Having observed these differences in the connection of brain neurons, they sought to understand the role of IKZF1 and IKZF2 in the lymphocytes of human patients.
To know the function is necessary to analyze their secretome: knowing what is released from the lymphocytes could help to unmask the molecular bridge between the immune system and central nervous system responsible of the observed neuronal changes. First, knowing the role of CD4+ lymphocytes, the most expected candidates could be cytokines, chemokines and growth factors. This is important because these signals could affect neural behavior and the neural network. They classified the human samples for both individual sexes in two groups: the double down regulated and the control (normal levels). Next, they cultured the human lymphocytes and harvested the culture medium (known as “supernatant”) that contains all the released products by the lymphocytes. They first did a protein expression screening (using a mass spectrometry technique) and cytokines and chemokines (signal messengers released by the lymphocytes) screening using a Luminex-based technique. Luminex is an immunoassay that precisely measures multiple analytes in one sample. They found an altered secretion IL-4, CXCL10, and CCL5 chemokines that could affect the neuronal synchronization and synaptic plasticity.
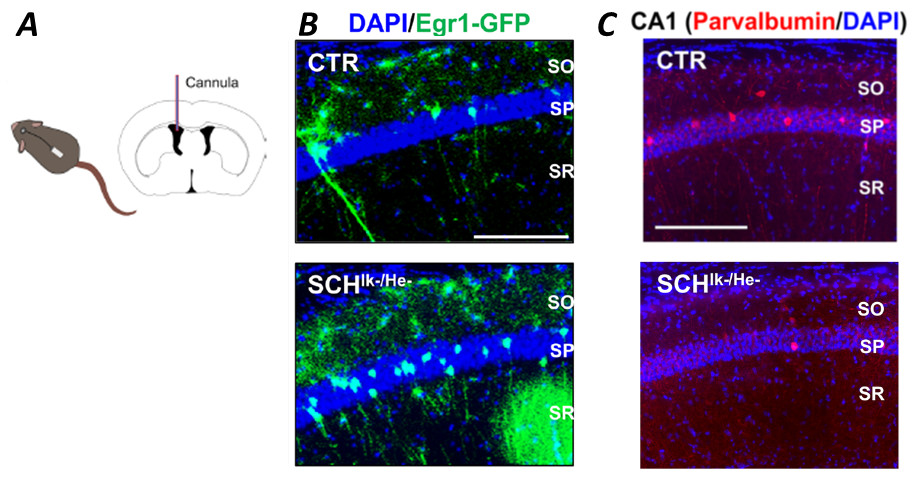
To corroborate the potential effect of the relased chemokines, they decided to test the secretomes in mouse hippocampal primary neurons (at day in vitro 7) using a 1:10 dilution. They evaluated the neuronal response analyzing the branching (interconnections of neurons in culture) and found a significant reduction of post-synaptic marker PSD-95, demonstrating a specific effects on the structural synaptic plasticity. Next, using the secretome of patients with schizophrenia they found a reduction of neuronal synchrony mimicking what observed in schizophrenia, demonstrating its role in the modulation of neuronal dynamics. To extrapolated the relevance into a more functional model, they used an in vivo experimental designin which their neurons activated by experience or relevant stimuli could be permnnently tagged (double mutant mice expressing Egr1-CreERT2 and
R26RCE). The secretome of control or schyzophrenic subjects was infused intracranially (into lateral ventricles of the brain of the animals) using osmotic pumps (Figure 1). This strategy allows a continuous administration up to maximum 20 days controlling the concentration, flow and the brain region exposed without need of animal handling (after the surgical operation). They used this window of time to do a broad behavioral characterization to assess the three dimensions of schizophrenia-like phenotypes. At the end of the experiment, they treated with 4-HT to induce recombination and permanent labeling of activated neural circuits (known as “engrams”). They found a reduction in sociability in the three-chamber social interaction test. Concerning the “novel object recognition test”, altered spine density in the CA1 pyramidal neurons was observed in the animals infused with the secretome of downregulated IKZF1 and IKZF2 groups.
In conclusion, they found that the reduction of IKZF1-IKZF2 in immune cells from schizophrenia patients disrupts communication with the brain, contributing to disease symptoms. This study suggests a novel molecular mechanism that could be key for developing immune-targeted therapeutic strategies, reinforcing the idea that schizophrenia is not just a brain disorder but a multisystem condition with a strong immune component.
References
- Davis J, Eyre H, Jacka FN, Dodd S, Dean O, McEwen S, Debnath M, McGrath J, Maes M, Amminger P, McGorry PD, Pantelis C, Berk M. A review of vulnerability and risks for schizophrenia: beyond the two hit hypothesis. Neurosci Biobehav Rev. 2016;65:185–94. doi:10.1016/j.neubiorev.2016.03.017 ↩
- Remington G. Book review: the schizophrenia spectrum. Can J Psychiatry. 2018;63:257–257. doi:10.1177/0706743718758042 ↩
- Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, Miller BJ. TNF-α and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr Res. 2018;199:281–4.
doi:10.1016/j.schres.2018.02.048 ↩
- Liemburg EJ, Nolte IM, Klein HC, Knegtering H. Relation of inflammatory markers with symptoms of psychotic disorders: a large cohort study. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;86:89–94. doi:10.1016/j.pnpbp.2018.04.006 ↩
- Busse S, Busse M, Schiltz K, Bielau H, Gos T, Brisch R, Mawrin C, Schmitt A, Jordan W, Müller UJ, Bernstein H-G, Bogerts B, Steiner J. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26:1273–9. doi:10.1016/j.bbi.2012.08.005 ↩
- Georgopoulos K, Winandy S, Avitahl N. The role of the ikaros gene in lymphocyte development and homeostasis. Annu Rev Immunol. 1997;15:155–76. doi:10.1146/annurev.immunol.15.1.155 ↩
- Raquel Martín-Ibáñez, Empar Crespo, Noelia Urbán, Solène Sergent-Tanguy, Cristina Herranz, Montserrat Jaumot, Manuel Valiente, Jason E Long, José Ramón Pineda, Celia Andreu, John L R Rubenstein, Oscar Marín, Katia Georgopoulos, Guadalupe Mengod, Isabel Fariñas, Oriol Bachs, Jordi Alberch, Josep M Canals. Ikaros-1 couples cell cycle arrest of late striatal precursors with neurogenesis of enkephalinergic neurons. J Comp Neurol. 2010 Feb 1;518(3):329-51. doi: 10.1002/cne.22215. ↩
- Ballasch, I., López-Molina, L., Galán-Ganga, M. et al. Alterations of the IKZF1-IKZF2 tandem in immune cells of schizophrenia patients regulate associated phenotypes. J Neuroinflammation 2024, 21, 326. doi: 10.1186/s12974-024-03320-3 ↩