Could regulators of the immune system have a role in schizophrenia?
Author: José R. Pineda got his Ph.D. from University of Barcelona in 2006. Since 2007 he has worked for Institut Curie and The French Alternative Energies and Atomic Energy Commission. Currently he is a «Ramón y Cajal» researcher affiliated to UPV/EHU and the Achucarro Basque Center for Neuroscience. He investigates the role of stem cells in physiological and pathological conditions.
In a new publication led by Dr. Albert Giralt, the researchers discovered the interaction between a transcription factor identified as “Helios” and a protein called “WDFY1” that could help elucidate the link between immunity and schizophrenia 1. Schizophrenia is an incurable mental illness characterized by relapsing episodes of psychosis. This disorder affects approximately 1% of the world’s population and requires lifelong treatment. Signs and symptoms can vary, but usually include delusions, hallucinations with speech, disorganized thoughts, social withdrawal, anhedonia, and behaviors. Over the years, it has been proposed that one of the factors contributing to schizophrenia could be the aberrant interaction of the immune system provoking alterations such as brain inflammation which in turn could contribute to schizophrenia. However, the molecular mechanisms are little known and genetic predisposition together with both immunological and neural alterations during development could trigger schizophrenic symptoms.
The role of the WDFY1 protein has been studied as a Toll-receptor (TLR3/4) scaffold/recruiting protein in the immune system 2 and in different oncogenic conditions. However, its function in brain remains poorly understood. In previous research led by Dr. Canals and by Dr. Giralt, they demonstrated that Helios, a transcription factor that classically acts as a potent regulator of immune-related molecular pathways, was also involved in the neurological development of the striatum and hippocampus 3. Both brain regions are known to be affected in patients with schizophrenia 4 . The approach with High Throughput RNAseq made it possible to identify the Wdfy1 gene (WD Repeat and FYVE Domain Containing 1) as the main target of Helios.
The WDFY1 protein is an adaptive protein for the Toll-like receptor signaling pathway 3 and 4 (TLR3 / 4) and, as such, mediates innate and adaptive immune responses. In the present study, using genetically-engineered animals with Helios gene removed (He-/- mice), they found an aberrant and sustainably regulated levels of the Wdfy1 gene. Furthermore, after a thoughtful analysis of the socio-cognitive and impulsive and dopamine-associated behaviors of He-/- mice they found several phenotypes similar to schizophrenia related to dysfunctions in the striatum and hippocampus.
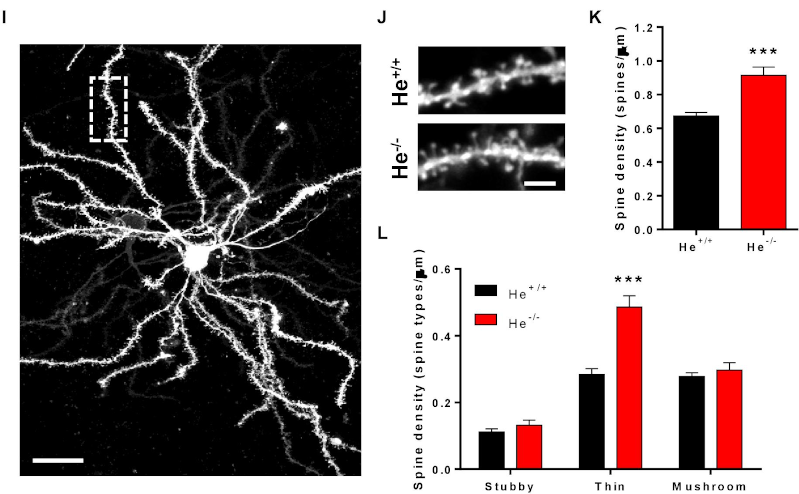
To validate the relevance of these observations with the disease, they analyzed the levels of WDFY1 protein in patients and showed an increase in the hippocampus and dorsolateral prefrontal cortex of schizophrenic patients, but not in the hippocampus of patients with Alzheimer’s disease with an associated psychotic disorder. These results highlighted the specificity of the relationship between the marker and the disease. To fully gain insight in the molecular mechanism they analyzed the synapses in the mouse model and found an increase in spine density in Medium Spiny neurons (MSN) with a decrease in the number and size of post-synaptic protein PSD-95 in a hippocampal region.
Interestingly, the observed phenotype also has been described in schizophrenic patients in previous studies 5 . In addition, the alterations observed in structural synaptic plasticity are associated with changes of the neuronal NF-κB levels, strengthening the link with the modulation of the immune response. All these results shows that He-/- mice are a valid model to study how alterations produced during the development of the central nervous system. Moreover, this new data pave the way to link the molecular pathways typically involved in immunomodulatory processes susceptible to trigger cumulative events able to culminate into neuropsychiatric disorders such as schizophrenic pathology.
Although more detailed molecular mechanisms are needed to understand the schizophrenia, new models for the discovery of drugs and drug repositioning could pave the way to characterize Ikaros and Helios family genes to move towards a possible cure for schizophrenia.
References
- Anna Sancho-Balsells, Veronica Brito, Belissa Fernández, Mónica Pardo, Marco Straccia, Silvia Ginés, Jordi Alberch, Isabel Hernández, Belén Arranz, Josep M. Canals and Albert Giralt. Lack of Helios During Neural Development Induces Adult Schizophrenia-Like Behaviors Associated With Aberrant Levels of the TRIF-Recruiter Protein WDFY1. Front. Cell. Neurosci., 14 May 2020 | https://doi.org/10.3389/fncel.2020.00093 ↩
- Hu YH, Zhang Y, Jiang LQ, Wang S, Lei CQ, Sun MS, Shu HB, Liu Y. WDFY1 mediates TLR3/4 signaling by recruiting TRIF. EMBO Rep. 2015 Apr;16(4):447-55. doi: 10.15252/embr.201439637. PMID: 25736436. ↩
- Giralt A, Brito V, Pardo M, Rubio SE, Marion-Poll L, Martín-Ibáñez R, Zamora-Moratalla A, Bosch C, Ballesteros JJ, Blasco E, García-Torralba A, Pascual M, Pumarola M, Alberch J, Ginés S, Martín ED, Segovia J, Soriano E, Canals JM. Helios modulates the maturation of a CA1 neuronal subpopulation required for spatial memory formation. Exp Neurol. 2020 Jan;323:113095. doi: 10.1016/j.expneurol.2019.113095. PMID: 31712124. ↩
- Lanz TA, Reinhart V, Sheehan MJ, Rizzo SJS, Bove SE, James LC, Volfson D, Lewis DA, Kleiman RJ. Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: a comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Transl Psychiatry. 2019 May 23;9(1):151. doi: 10.1038/s41398-019-0492-8. ↩
- Matosin, N., Fernandez-Enright, F., Lum, J. S., Engel, M., Andrews, J. L., Gassen, N. C., et al. (2016). Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr. 2:16022. doi: 10.1038/npjschz.2016.22 ↩
1 comment
[…] Gaixotasun kronikoa da eskizofrenia eta bere atzean dauden mekanismoak ez daude batere argi. Sistema inmunitarioak zerikusia duela izan liteke jarraitu beharreko pista. Rosa García-Verdugoren Could regulators of the immune system have a role in schizophrenia? […]