Mimicking Nature’s light-harvesting efficiency: the case of sulphur-bridged terthiophene dimers
Mimicking Nature’s light-harvesting efficiency: the case of sulphur-bridged terthiophene dimers
In nature, light harvesting organisms make extensive use of energy and electron transfer between adjacent molecules. Thus, in the photosynthetic cell of an algae, bacterium, or plant, there are light-sensitive molecules called chromophores, which contain a π-conjugated system (a system with alternate single and double bonds), arranged in an antenna-shaped structure named a photocomplex. When a photon is absorbed by a chromophore, it is converted into a quasiparticle referred to as an exciton, which jumps from chromophore to chromophore towards the reaction center of the photocomplex, a collection of molecules that traps its energy in a chemical form that makes it accessible for the cell’s metabolism.
A great deal of work has been focused on synthesizing electron donor/acceptor pairs to mimic these efficient natural systems. The most common approach is to unite an electron-rich donor with an electron-deficient acceptor using a π-conjugated linkage or “bridge.” Separation of the donor and acceptor by such a bridge can enable charge transfer over large distances, limiting charge recombination. While remarkable photovoltaic performance has been achieved using this approach, these asymmetric systems only partially mimic the naturally occurring photosynthetic reaction centers, where symmetric chromophore pairs have a central role in controlling excited-state dynamics.
The rational design of symmetrically bridged chromophore dimers, also called bichromophores, has attracted considerable theoretical and practical interest. For example, bichromophoric systems have also found applications in organic light emitting diodes (OLEDs) and high-efficiency blue OLEDs were fabricated using SO2-bridged symmetric bichromophores.
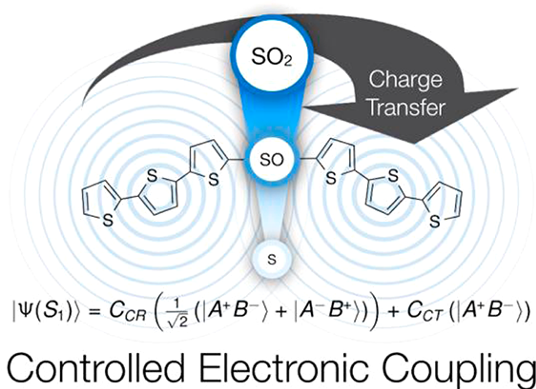
Recently, it was demonstrated that bridging two conjugated chromophores symmetrically about a sulphur atom results in systematic enhancement of the photoluminescence by oxidizing the bridging sulphur. Increasing the oxidation state of the sulphur bridge also resulted in pronounced red-shifts of the photoluminescence spectra in polar solvents, an indication of increasing charge transfer character. These experiments were done using terthiophenes as chromophores. Thiophene is a heterocycle formed by four carbon and one sulphur atoms; terthiophene is a molecule consisting of three thiophenes linked.
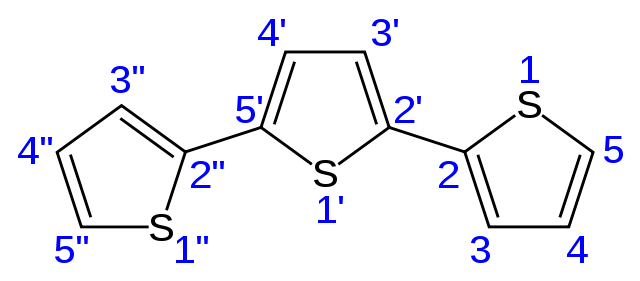
The observation that simply linking two terthiophenes together via a sulphur atom could lead to new charge transfer excited states and increase the photoluminescence yield by an order of magnitude raised several questions regarding the origin of the changes in spectroscopic behavior. The first question concerns the role of the sulphur linker: Does it actively participate in the electronic states, and why does its oxidation state have such a large effect? Related to this question, one may ask whether the second chromophore plays an important role. If the charge transfer state involves only one of the terthiophene groups and the bridging sulphur, then perhaps the second terthiophene is not even necessary. The question of electronic structure is intimately related to the excited-state dynamics: How is the charge transfer state formed and why is its relaxation different from that of a single chromophore?
As this class of sulphur-bridged terthiophene dimers, with potential applications in organic electronics, provides an ideal model system in which to study fundamental questions about the excited-state behavior in symmetric bichromophore systems, a group of researchers, including David Casanova, an Ikerbasque Research Fellow working at the Kimika Facultatea UPV/EHU and DIPC, focused on answering this questions and combined spectroscopy and computation to obtain a comprehensive picture of the photodynamics of sulphur-bridged terthiophene dimers. The results1 are published in the Journal of the American Chemical Society.
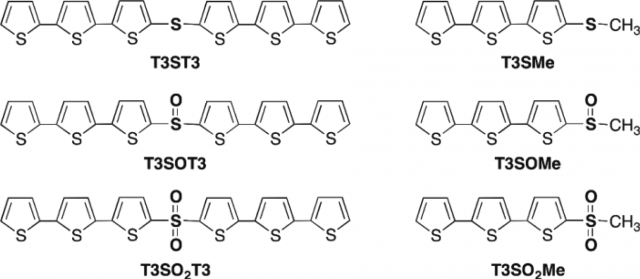
The researchers used optical spectroscopy (steady-state and time-resolved) and electronic structure theory to develop a complete picture of the photophysics of a group of terthiophene derivatives (Figure 3). They found that the bridge itself does not significantly affect the excited-state structure, but the oxidation state of the sulphur bridge mediates the amount of electronic coupling between the two terthiophene chromophores.
Based on computational results, the researchers propose a mechanism in which electrostatic screening by lone pairs on the sulphur linker controls the charge transfer character of the excited-state wave function. This suggests that it is possible to use electron density on the bridge to mediate intramolecular interactions and tune the electronic coupling between identical chromophores without altering the dimer geometry or solvent polarity.
These results not only reveal the origins of the novel properties of a specific class of terthiophene bichromophores but also provide new directions for the design of symmetric chromophore systems that may find applications in fields ranging from artificial photosynthesis to organic electronics.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Chad D. Cruz, Peter R. Christensen, Eric L. Chronister, David Casanova, Michael O. Wolf, and Christopher J. Bardeen (2015) Sulfur-Bridged Terthiophene Dimers: How Sulfur Oxidation State Controls Interchromophore Electronic Coupling JACS DOI: 10.1021/jacs.5b05457 ↩