Will your own immune system be the cure to your own cancer?
Will your own immune system be the cure to your own cancer?
Recently a deal between the biopharmaceutical companies Gilead and Kite Pharma was announced. Under the agreement, Gilead will buy Kite for a stunning USD 11 billion, shifting the focus of Gilead from infectious diseases to the field of oncology, just like that. But why write a post on MI about such apparently business-related issue? Kite Pharma is a small company developing the so-called Chimeric Antigen Receptor T-Cell Therapy or CART, which is a novel type of therapy against cancer (some people call it the fifth pillar), while Gilead is one of the biggest biotech companies in terms of market value (nearly $100 billion or 10% of Spanish GDP per year), but working in a completely different area of research and development. But still, why should this be good news? There are two direct factors contributing to such optimism. The first one is that the pipeline from Kite Pharma is now owned by a company with cash worth USD 36 billion, which means they have the financial muscle to progress through the costly clinical trials needed to successfully commercialize such bio-therapies. Second, it means that CART is not just an unproven experimental eccentricity, but a therapy that is worth investing in, and given Gilead´s previous success with both Sovaldi and Harvoni for Hepatitis C, that may mean a lot.
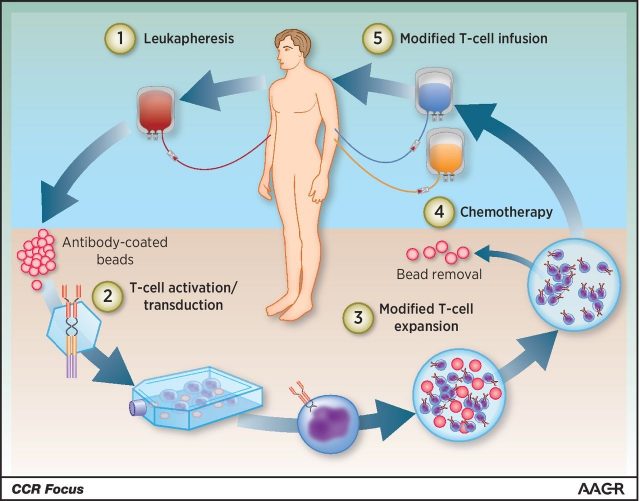
So why is CART so promising? This therapy belongs to the area of immunotherapy, which involves the use of the patient´s own immune system to fight cancer, with best results achieved on haematological-related malignancies (although fast improvement is also happening in solid tumours too). Given its effectiveness and specificity, it becomes clear that once this technology is fully developed and improved it may mean there is real hope for people suffering (and dying) from cancer. The trick is that a particular type of white blood cell called T-lymphocyte (crucially involved in cell-mediated immunity) are engineered adding to them synthetic receptors which can recognize antigens that are only expressed in cancer cells. To do so, T-lymphocytes are collected from the patient´s blood, isolated and (importantly) activated before they are reprogrammed by introducing a construct containing the DNA sequence coding for the desired chimeric receptor which will recognize the antigen of choice present on the cancer cells to be targeted. Generally, these constructs are inserted using retroviruses. After a round of modified T-lymphocyte expansion to exponentially increase the number of cells, these are re-infused to the patient (Figure 1). Gilead says the vein to vein time is somewhere in the range of 17 days, thus making it a fairly fast option for cancer patients.
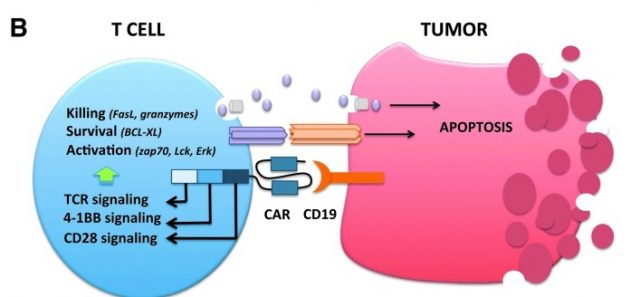
To date, the CAR from which most clinical data is available is CD19-specific Car T cells. CD19 is a protein expressed in another type of white blood cells called B-lymphocytes, involved in different roles such as secretion of antibodies, presentation of antigens or cytokine secretion. CD19 is specifically expressed in B-lymphocytes related malignancies including B-cell acute lymphoblastic leukaemia (in bone marrow), chronic lymphocytic lymphoma, diffuse large B-cell lymphoma and follicular lymphoma (all those generating from secondary lymphoid organs), thus making it a great target as antigen1. Complete response rates of nearly 90% have been reported from both paediatric and adult patients suffering from the above, but not with just CD19 CAR T-cells, but cells expressing CD28 or a 4-1BB costimulatory intracellular domains along with CD19 (Figure 2). Addition of these cytoplasmic costimulatory domains greatly improves T-lymphocyte antitumor activity by augmenting cell persistence and proliferation rates. These cells comprise the second generation of CART with subsequent generations aiming at adding-up costimulatory domains to further enhance persistence. This is one of the most important factors weighing on the success of the therapy, as the longer the T-cells can survive, the more likely will be having a favourable outcome. Still, a prolonged persistence is not the only downside from CART, unfortunately. Both Kite Pharma and a competing company called Juno Therapeutics have had few fatal cases during the progression of clinical trials as a consequence of cerebral oedema. There is no clear answer as to whether the deaths are directly linked to the therapy, but a recent study shows evidence that CAR T-cells may enter the brain through the blood-brain barrier and trigger excessive cytokine release, at least in one patient. Intracranial edema was subsequently observed by researchers, hence providing a potential explanation 2.
Despite such events, the FDA granted approval last 30th of August to the first gene therapy available in the US. Kymriah (tisagenlecleucel) from Novartis is a cell-based immunotherapy targeting certain paediatric and young adult patients with a subtype of acute lymphoblastic leukemia (ALL). However, it also comes with a risk evaluation and mitigation strategy mainly because there is the possibility of suffering cytokine release syndrome 3. Authorities believe the positives may surpass negatives, but as it seems to be the case lately (see my previous post from DMD), the FDA goes ahead and decides to try in the general population.
Besides further consideration, it seems clear that immunotherapy is a research arena destined to revolutionize the field of cancer. Gilead´s large investment effort buying Kite Pharma serves as proof. It is really important to put in place strategies aiming at prevent this horrible disease, but it is also paramount to have a mitigation plan once the disease strikes inadvertently. A lot of unnecessary suffering would be spared, that is for sure.
References
- Maus MV, June CH. Making Better Chimeric Antigen Receptors for Adoptive T-cell (2016) Therapy. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-15-1433. ↩
- Hu Y, Sun J, Wu Z, Yu J, Cui Q, Pu C, Liang B, Luo Y, Shi J, Jin A, Xiao L, Huang H. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. (2016) J Hematol Oncol. doi: 10.1186/s13045-016-0299-5. ↩
- FDA News Release (August 2017) FDA approval brings first gene therapy to the United States ↩