Immune-based therapies to fight leukemia
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Acute leukemia is characterized by the proliferation and accumulation of hematopoietic progenitor cells (which are able to differentiate to create specialized cell types) in the bone marrow that impede normal blood production. Depending on the cell population affected, it can be classified in Acute Lymphoblastic Leukemia (ALL) and Acute Myeloid Leukemia (AML). ALL starts in cells that become lymphocytes (B or T lymphocytes) and is the most common form of cancer in children, accounting for approximately 30% of all pediatric cancers. Of these, approximately 85% of cases are B-ALL (caused by the accumulation of immature B-cell progenitors in the bone marrow) 1. On the contrary, Acute Myeloid Leukemia (AML) begins in early myeloid cells, which following specific maturation steps, become white blood cells, red blood cells or platelets and is one of the most common forms of acute leukemia in adults (approximately 80% of leukemias in this group) 2.
The success of standard chemotherapy in the treatment of acute leukemia is highly dependent on the type of leukemia as well as on the genetic alterations present in leukemic cells. Thus, therapies taking advantage of a patient’s own immune system emerged as a novel and effective means of treating cancer. In an interesting paper published by Witkowski and co-workers, the authors discuss the use of immune-based therapy as a useful tool to fight leukemia, giving higher relevance to three different immune-based treatments: the use of chimeric antigen receptor (CAR) T cells, bi-specific T cell engagers (BiTEs) and immune checkpoint blockade (ICB) in the treatment of acute leukemia .3. Here I summarize each one of these strategies:
Chimeric antigen receptor (CAR-T cells):
This strategy focus on the generation of genetic constructs that can be incorporated into a patient’s own T cells (generating the CAR-T cells) to help them to recognize and fight cancer cells. To do this, firstly T cells from a patient with leukemia are removed and cultured in vitro. Then, the CAR genes that are directed against the patient’s leukemia type are inserted and the modified T cell population is expanded in vitro and injected into the patient. The genetic modification allow to the engineered T cells to eliminate antigen-expressing target (cancer) cells. An ideal CAR-T target candidate should be widely expressed on malignant cells, to ensure eradication of the disease, and be rarely expressed on healthy cells, to avoid treatment-induced toxicity. Prior to CAR infusion, patients are treated with agents that profoundly deplete lymphocytes 4 .
CAR-T cell therapy has been highly successful in the treatment of relapse B-ALL using CD19 antigen as target candidate. Contrary to the successful use of CD19-CAR-T treatment in B-ALL 5, no ideal target has been identified in AML to date.
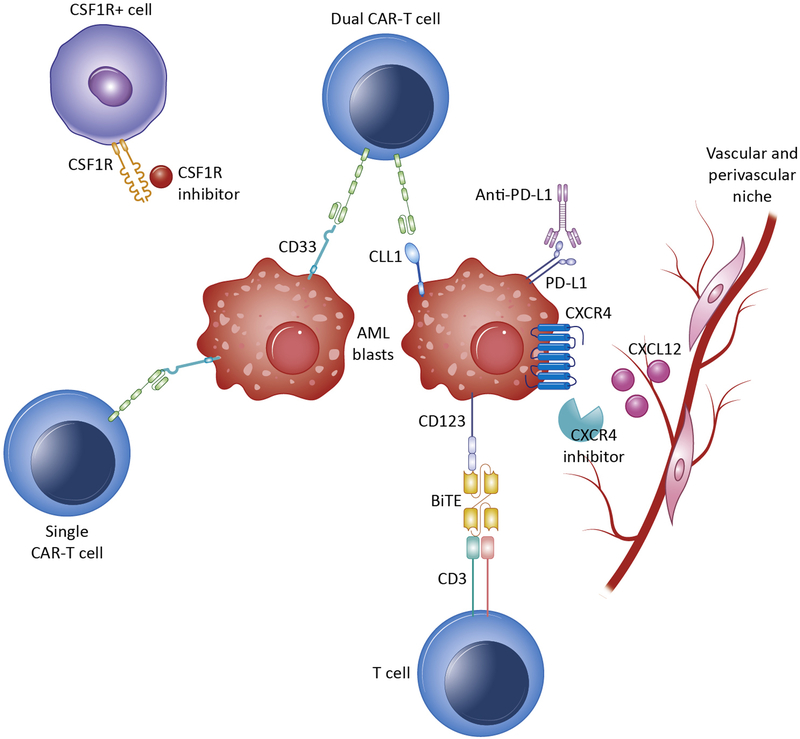
Bi-specific T cell engagers (BiTE / BiKE):
BiTE are bi-specific monoclonal antibodies (laboratory-made proteins that mimic the immune system’s ability to fight to something dangerous), that simultaneously target T cells and tumor cells, thus forming a direct physical link between them 6. This leads to killing of the tumor cell by the T cell (6). BiTE are an attractive treatment because they represent a “ready to use’’ strategy, unlike CAR-T that have to be adapted for each patient. Another attractive possibility is targeting of NK (natural killer) cells (a type of lymphocyte that provide a rapid immune response) in the tumor microenvironment, using bi-specific killer cell connectors (BiKE). These are bi-specific antibodies targeting a tumor cell antigen and CD16, expressed on NK cells (BiKE) 7. Linkage of tumor and NK cells induces tumor cell killing by the NK cells.
Immune checkpoint blockade (ICB):
It is already known that tumoral cells can induce an immunosuppressive microenvironment to prevent their elimination by the immune system. A key feature of this strategy is the exhaustion of T cells mediated by expression of immune checkpoint molecules in tumor cells. Checkpoint molecules are regulators of the immune system, specifically on infiltrating T cells. The mechanism of T cell exhaustion is characterized by poor effector function and it is provoked by prolonged exposure to antigen stimulation. For example, and important axis for T cell exhaustion in the tumor microenvironment is the PD-1/PD-L1 interaction: PD-1 is expressed on T cells and interacts with its ligand PD-L1 that is expressed on tumor cells. This permanent interaction induces T cell exhaustion 8. Thus, treatment with antibodies blocking the PD-1/PD-L1 interaction leads to re-invigoration of T cells in the tumor microenvironment and is remarkably effective in solid tumors 9. At present, clinical trials that enrolled patients with different hematopoietic malignancies including AML showed that anti-PD-1 agents could have potential therapeutic effects in the patients.
In summary, immune-based treatments have emerged as effective treatment for chemoresistant B-ALL and AML and take advantage of a person’s own immune system to help kill cancer cells avoiding immune system rejection. For this reason, at present, different approaches are being developed to enhance the effectiveness of immune-based treatments.
References
- Stephen P Hunger, Charles G Mullighan. Acute Lymphoblastic Leukemia in Children. N Engl Med. 2015; 373: 1541-52. PMID: 26465986 DOI: 10.1056/NEJMra1400972 ↩
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leulemia: a comprehensive review and 2016 update. Blood Cancer J. 2016; 6: e441. PMID: 27367478 DOI: 10.1038/bcj.2016.50 ↩
- Witkowski MT 1 , Lasry A 2 , Carroll WL 3 , Aifantis I. Immune-Based Therapies in Acute Leukemia. Trends Cancer. 2019; 5: 604-618. PMID: 31706508 DOI: 10.1016/j.trecan.2019.07.009 ↩
- Ruella M et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018; 24: 1499-1503. PMID: 30275568 DOI: 10.1038/s41591-018-0201-9. ↩
- Lee DW et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015; 385: 517-528. PMID: 25319501 DOI: 10.1016/S0140-6736(14)61403-3 ↩
- Bene MC et al. Immunophenotyping of acute leukemia and lymphoproliferative disorders: a consensus proposal of the European LeukemiaNet Work Package 10. Leukemia. 2011; 25: 567-574. PMID: 21252983 DOI: 10.1038/leu.2010.312 ↩
- Wiernik A et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013; 19: 3844-3855. PMID: 23690482 DOI: 10.1158/1078-0432.CCR-13-0505 ↩
- Ribas A, Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018; 359: 1350-1355. PMID: 29567705 DOI: 10.1126/science.aar4060 ↩
- Jalili-Nik M, Soltani A, Mashkani B, Rafatpanah H, Hashemy SI.PD-1 and PD-L1 inhibitors foster the progression of adult T-cell Leukemia/Lymphoma. Int Immunopharmacol. 2021; 98: 107870. PMID: 34153661 DOI: 10.1016/j.intimp.2021.107870 ↩