BPA and pregnancy: diabetes for you and your kids
BPA and pregnancy: diabetes for you and your kids
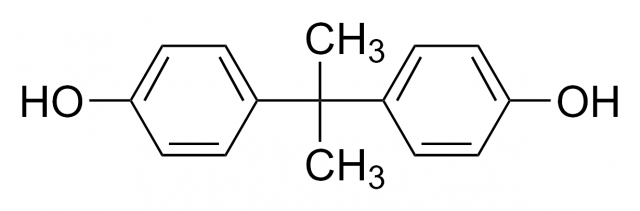
«BPA-Free», «No Bisphenol A» and similar labels have become a sort of seal of quality for those products where they are on display, especially containers and baby products. But, what is bisphenol A? In 1936 bisphenol A (BPA) was identified as a synthetic estrogen, this is, an artificial compound that shows estrogen-mimicking action. Estrogens are hormones that exert a wide range of actions in the organisms, but they are principally related to the menstrual cycle and pregnancy. Many studies have linked bisphenol A to hormones ever since its estrogenic effects were proved in the last century.
However, BPA is mainly used to make plastics, especially plastic containers, for it is the base molecule in polycarbonate production (widely used to make baby and water bottles and food containers) as well as in some metal-based food cans. Besides, it is employed to harden PVC plastics and it is also a key component in thermal paper on sale receipts.

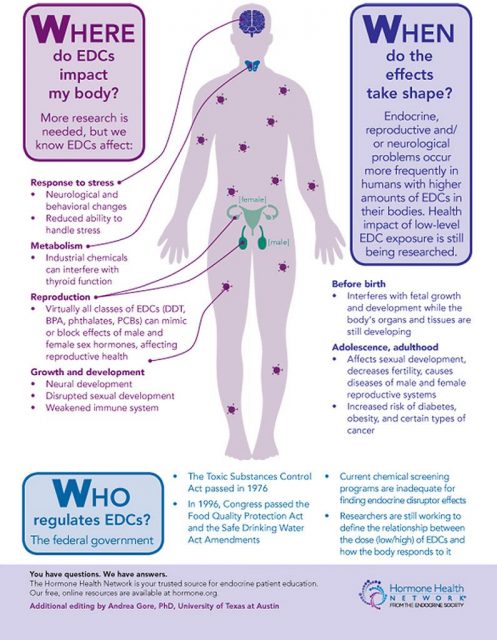
With time it was demonstrated that BPA was capable of leaching from the lining of plastic containers into food and beverages. In fact, a scientific study detected that 92.6% of U. S. citizens had detectable levels of bisphenol A in their urine. Other reports give account of BPA detections in amniotic fluid, blood, the placenta or breast milk, which indicates that infants may be exposed to this compound via their mothers.
Early studies on the possible health risks of BPA were focused on its carcinogenicity and its impact on the reproductive system. During the last decade additional effects were proposed, such as pathologies associated with the development of the nervous system, alterations on the thyroid gland, diabetes mellitus, obesity and cardiovascular diseases. BPA action as an endocrine disruptor occurs primarily when there is an exposure during pregnancy, affecting fetal development. This period is of great vulnerability for both the fetus and the mother. Studies in animal models have analysed the effect of BPA on fetal development and the consequences for the phenotype later in life. Less is known about adverse effects that endocrine disruptors may have on the mothers after childbirth and in the future.
The research line of Dr. Nadal´s group aims to investigate the exposure to endocrine disruptors, mainly BPA, on the etiology of diabetes mellitus type 2. But, why diabetes? Pregnancy implies significant anatomical and physiological alterations (hormonal changes), but it also affects general metabolism. In this respect, one of the major changes is observed in glucose metabolism. Glucose is a key nutrient for fetus development and for milk production, therefore, glucose blood levels must be higher during pregnancy in order to respond to these new requirements. In late pregnancy, glucose production is enhanced and glucose uptake by muscle or adipose tissue progressively decreases. Both factors enable an increased availability of glucose for the fetus.
Tissues incorporate glucose from the blood as a response to insulin: when organs detect insulin in the blood, they consume glucose. During pregnancy, muscle and adipose tissue are less reactive to insulin, this is, they become insulin resistant (they behave like there was a type 2 diabetes condition). In order to compensate all these changes, maternal pancreas adapts by increasing insulin secretion (as a consequence of producing more beta cells). If this pancreatic adjustment fails and there is not enough insulin, blood glucose levels raise significantly. In this situation, we might be facing gestational diabetes.
Insulin resistance in peripheral tissues seems to be related to gestational hormonal changes. Thus, estrogen maternal serum levels rise, having an impact in pancreatic insulin synthesis and secretion. As BPA is an artificial estrogen, could it be associated with gestational diabetes?
Dr. Nadal´s group showed1 that mice treated with BPA for 8 days during pregnancy (mice have a 19-21-day gestation period) present metabolic features close to gestational diabetes: high blood glucose levels, insulin resistance and increased body weight. These effects were visible in the gestational period and also 4 months postpartum. After that moment the symptoms were more severe, which means that BPA treatment altered maternal glucose metabolism later in life. This effect was not observed when female nonpregnant mice were treated with BPA. Therefore, bisphenol A modifies glucose metabolism in pregnancy hormonal conditions.
Early studies already proved that 6-month old male mice offspring exposed in utero showed the same diabetes symptoms as their mothers, namely higher glucose levels and insulin resistance. These effects were not observed in the female mice. Being BPA a synthetic estrogen it may have deeper effects on male mice, as these present lower estrogen levels. On the other hand, it has been proved that BPA exposure during pregnancy predisposes male mice to have an increased pancreatic beta cells growth, which may induce impaired glucose tolerance later in life.
An important aspect to be considered in this type of toxicity studies is the concentration of the substance. Sometimes these studies are performed using concentrations that far exceed those normally found in the food consumed by people or even those recommended by the competent authorities, so there is no cause for public alarm. At excessive doses all compounds are toxic (“the dose makes the poison”). Are the experiments discussed below a paradigmatic example of this matter? The observed effects are due to extremely high concentrations of the substance? No, it is not the case.

Administrations are the ones in charge of regulating the highest dose of a substance that does not cause unacceptable side effects. These legal requirements are precisely seeking to avoid the development of adverse effects derived of the extensive use of a given chemical compound. In this context, FDA (the U. S. Food and Drug Administration) recommends a safe reference dose for BPA of 50 μg/kg/ bw/day. The study of Dr. Nadal’s group was performed at a concentration 5-fold lower (10 μg/kg/bw/day), which is the usual normal amount of BPA found in the environment. Thus, the results obtained here are of great relevance because they occur at ordinary concentrations, beneath the threshold of official recommendations. These dosage instructions are constantly adapted to the new experimental findings. In this context, the European Chemicals Agency (ECHA) recently recognised bisphenol A as a substance of very high concern.
References
- Paloma Alonso-Magdalena et al (2015) Bisphenol-A Treatment During Pregnancy in Mice: A New Window of Susceptibility for the Development of Diabetes in Mothers Later in Life Endocrinology doi: 10.1210/en.2014-1952 ↩
6 comments
[…] diabetesaren antzeko sintomatologia eragin lezake, bai amarengan bai umearengan. NuRCaMein-en BPA and pregnancy: diabetes for you and your kids […]
[…] El bisfenol-A (BPA) es un disruptor endocrino, es decir, una sustancia capaz de alterar el equilibrio hormonal. La exposición al mismo durante el embarazo provocaría sístomas parecidos a la diabetes, tanto en la madre como en el feto. NuRCaMein […]
“Besides, it is employed to harden PVC plastics”. The lack of rigor of such statement just starting the paper makes me doubt on the whole article. It would have been better to write: “Besides, it WAS employed to thermally stabilise PVC plastics”. In fact, ECHA does not mention PVC in his BPA dedicated page: https://echa.europa.eu/chemicals-in-our-life/hot-topics/bisphenol-a.
I would appreciate your permission to use some of the pictures posted on your website on the effect of BPA on human health. I will use them in writing a popular science article and acknowledge the source. Thanks
[…] Read the complete article… […]
[…] Check our latest article in Mapping Ignorance: BPA and pregnancy: diabetes for you and your kids. […]