How maternal vaginal microbiome can bring you to madness
Maternal and paternal stress have been proved to be critical aspects of off-spring brain development. High levels of stress on pregnant mothers can alter both placental and embryonic gene expression patterns, misprogramming the brain of the newborn towards psychiatric disorders such as anxiety or depression. At the same level, paternal stress alters microRNAs and other molecules of the spermatozoid, increasing the risk of disorders in the offspring.
These effects are linked to epigenetic dysregulations and initiate a completely new field of research, focused on transgenerational effects of maternal/paternal life experiences on neurodevelopment of the next generation. It is now clear that the life aspects of our parents can interfere with our development, predisposing us to different diseases in adulthood.
During the last years, the scientific community has also identified that gut microbiota has an important role in brain development. The human gastrointestinal tract is inhabited by 1×1013 to 1×1014 microorganisms — more than 10 times that of the number of human cells in our bodies. Although still not 100% clear how the brain and gut microbiota talk, it is evident that this conversation is through the nervous and immune systems, and metabolism. Many of the research performed on the gut microbiome-brain interaction has focused on how stress is able to modulate gut microbiome composition through the activation of the hypothalamic-pituitary-adrenal axis (HPA axis). Several examples have shown that long-term sustained HPA activity evoked by stress exposure may disrupt the microbiome of our body. For example, maternal separation, that alters the HPA axis in a long-term way, reduces in some monkey species the amounts of key lactobacilli. However, the exact consequences of such alterations remain confusing and not yet completely associated with brain function.
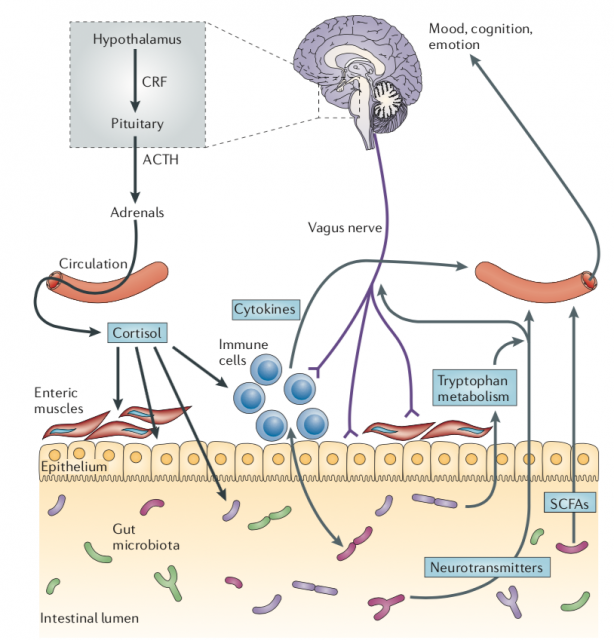
One example of how microbiota may alter brain function occurs in Alzheimer’s disease, where it has been described that alterations on gut microbiome, specifically a reduction on some bacterial phylum such Firmicutes (where belong genera like Bacillus, Lactobacillus or Clostridium) or Bifidobacterium (phylum Actinobacteria 1. However, like most human studies it is difficult to understand if that microbiome alteration is a cause or a consequence of the disorder. Like Alzheimer’s disease, other human disorders have been associated with microbiome, especially depression and anxiety. In germ-free mice, a powerful approach to study the microbiome, mild stress exposure (a model for depression/anxiety) resulted in an exaggerated HPA axis response, compared with mice with normal microbiota. This altered response was completely reversed by colonization with Bifidobacterium infantis 2.
The new information provided by Jašarević et al 3, from the group of Tracy Bale in the seminal paper “The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus” brings completely new evidence on the transgenerational transference of microbiome, that is able to modulate our brain function. In a series of experiments, the authors demonstrated that the transplantation of the vaginal microbiome from stressed mothers to normal pups, born by cesarean section in order to not have contact with their own mother vaginal microbiota, mimicked partially the effect observed in those pups born from stressed mothers. This suggests that maternal stress altered the vaginal microbiota of the mother that passed to the offspring and influenced their brain development. The maternal vaginal microbiota helps to colonize the gut of the offspring, aiming to stimulate immune and metabolic process in the pups.
A deeper analysis identified several bacterial species altered by maternal stress on gut microbiota of the offspring (through vaginal microbiota transference). Among them, Escherichia coli, Streptococcus acidominimus and Streptococcus thoraltensis and Peptococcaceae were altered in manipulated pups, with similar levels to pups normally born after maternal stress.
Although the stressed phenotype could not be reverted in normally born stressed pups (with the transference of non-stressed microbiome) the data provided suggest that microbiome manipulation may be a powerful therapeutical target in specific child populations, helping susceptible kids to cope better with stressful situations in adulthood. Also, suggest how challenging and intricate is our relationship with the environment, internal and external, and how essential is for our normal development. Both our life experiences and our gut microbiota, among others, are affecting our brain function, programming toward a health or disease adulthood. But all that scientific evidence also indicate another thing, the ability of our brain to control the whole body, including the microbiota helping it to develop and function. The brain, the complex machine responsible for making us what we are, is still far from being understood.
References
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE (2017). Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 19;7(1):13537. doi: 10.1038/s41598-017-13601-y ↩
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol; 558(Pt 1):263-75. doi: 10.1113/jphysiol.2004.063388 ↩
- Jašarević E, Howard CD, Morrison K, Misic A, Weinkopff T, Scott P, Hunter C, Beiting D, Bale TL (2018). The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat Neurosci. doi: 10.1038/s41593-018-0182-5 ↩