Vitamin D to protect from SARS-CoV-2 infection?
Vitamin D to protect from SARS-CoV-2 infection?
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Since COVID-19 disease arrived in our lives at the beginning of last year, we are giving increased importance to our immune system status. We are worried about if it is working properly and can attack correctly the SARS-CoV-2. For this reason, lot of people are started to take food supplements containing vitamin D. In fact, insufficiency or deficiency of vitamin D is a highly prevalent global problem, affecting over a billion people worldwide. But, why vitamin D would be so important to protect ourselves and fight the virus?
On one hand, it has been shown that vitamin D deficiency co-exists in patients with COVID-19 and together with the increased age and the presence of pre-existing illness, its deficiency seems to be linked to severe COVID-19 disease 1. On the other hand, recent findings show that vitamin D is able to enhance the innate immunity acting on different fronts to combat SARS-CoV-2 2. So, this would be the answer to the question above, and interestingly, Peng MY and co-workers collected the different aspects in which vitamin D is involved in the immune system response 3.
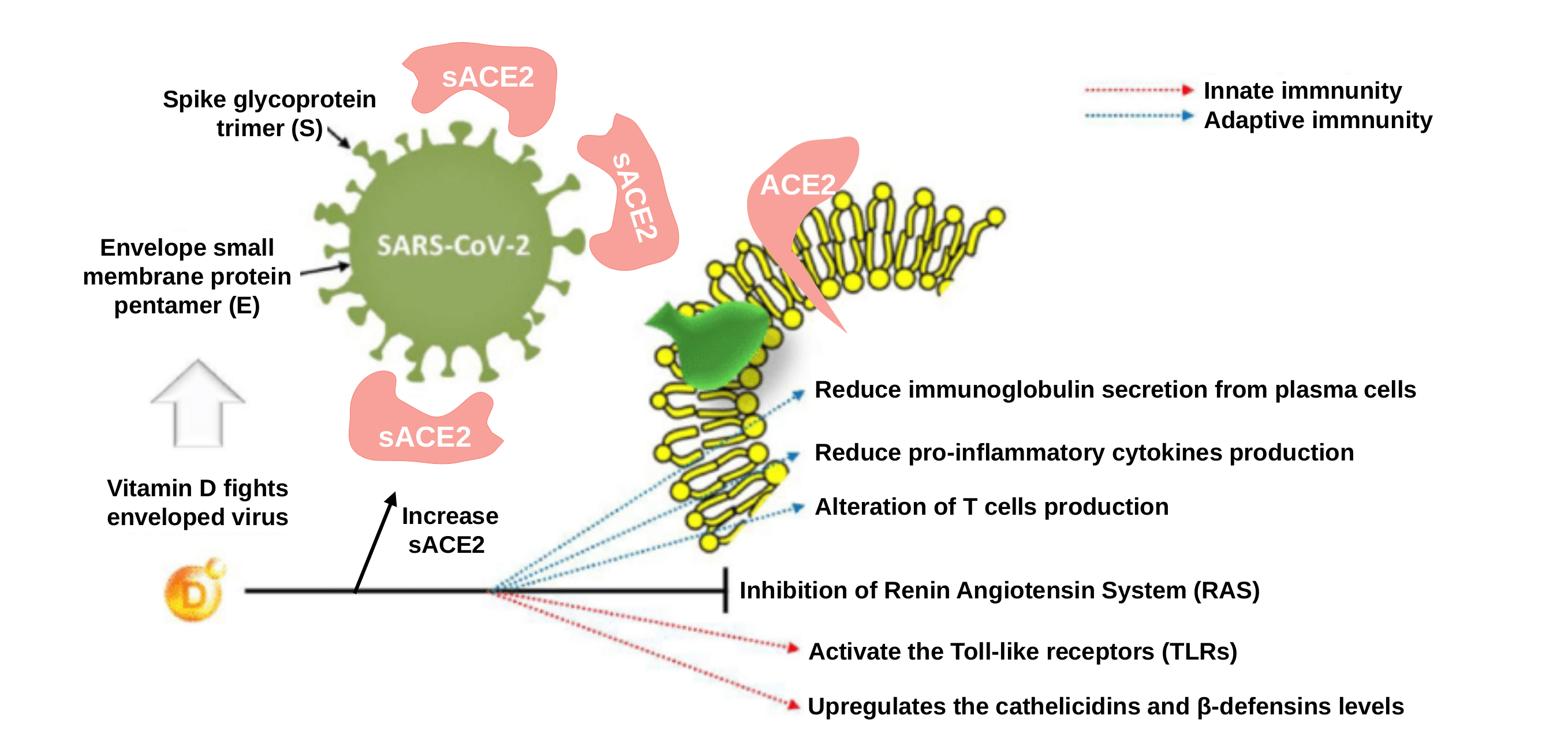
Firstly, the authors focus on the antiviral activity of vitamin D and its involvement in innate immune response. The promotion of antiviral immunity by vitamin D involves various mechanisms, such as the induction of two molecules: a peptide called “cathelicidin” and a protein called “defensin”. Interestingly, these molecules can block viral entry into the cells 4 and suppress the capability of the virus to produce more viral particles 5. Another property of vitamin D is that it acts through the promotion of autophagy 6, a fundamental biological process that maintains cellular equilibrium through the elimination of damaged cells organelles and proteins firstly encapsulated by intracellular membranes and then subsequently degraded. Besides, autophagy is also an essential mechanism by which cells respond to viral invasion. Specifically, autophagic encapsulation packages viral particles for lysosomal degradation and subsequent antigen presentation and activation of adaptive antiviral immune responses 7.
Next, Pen My and co-workers summarize the influence of vitamin D in adaptive immunity regulation. The adaptive immune system is initiated by the activation of antigen-presenting cells (such as static dendritic cells mainly located in the lymph nodes and spleen or the mobile macrophages, the “police” of our body) which in turn alert and activate the antigen-recognizing cells (that are T lymphocytes and B lymphocytes, the “army” of our body), which are major determinants of the immune response 8. The mechanism through the vitamin D is able to stimulate the cells of our immune system is using the active form of vitamin D that is also known as “calcitriol”. To achieve that, cells express the vitamin D-activating enzyme CYP27B1 and the vitamin D Receptor (VDR), which then utilize the circulating inactive vitamin D to convert it into calcitriol. Calcitriol directly modulates inflammatory cytokines (small proteins that act as messengers of our immune system and its surrounding tissue). The cytokines are dependent on the activity of a protein complex (present in numerous types of cells) known as NF-κB. Calcitriol regulates cytokines by blocking NF-κB activation via the upregulation of the NF-κB inhibitory protein called IκBα 9.
At this moment, it is interesting to describe one subpopulation of special relevance of T lymphocytes: the “helper T cells”. This subpopulation has distinct subtypes, including Th1, Th2, Th3, Th17, or TFH. Each of these subtypes secretes a different panel of cytokines that drives the immune response in a specific direction. In general, these lymphocytes “orchestrate and coordinate among them and our army” activating B cells to convert them into plasmatic cells (that produces high amounts of antibodies) and activating our macrophages (“police” in charge to destroy ingested microbes and also help activate cytotoxic T cells to kill infected target cells). The active vitamin D calcitriol acts on different fronts: i) inhibits the activation of Th1 cells and cellular immune responses related to tissue destruction; ii) promotes the association of Th2 cells with the antibody-mediated response; and iii) also mitigates inflammation and tissue damage by inhibiting the activation of pro-inflammatory immune Th17 cells 10 1112.
Concerning the rest of cell of our body, the majority of them express the “Toll-Like Receptors” (TLRs). These receptors are transmembrane proteins that recognize conserved molecular motifs of viral and bacterial origin and initiate innate immune responses. TLR3 recognizes viral double-stranded RNA and is primarily involved in viral defense. For example, it has been demonstrated that vitamin D attenuate the expression of IL-8 (a cytokine involved in the recruitment of neutrophils) to the site of damage or infection in respiratory epithelial cells by the formation of double-stranded RNA-TLR3 complexes 13.
The other interesting receptor directly linked to vitamin D is the ACE2 receptor. ACE2 is the Angiotensin-converting enzyme 2, a carboxypeptidase that was rapidly identified as the critical functional receptor for SARS-CoV-2. ACE2 is also well-known as a negative regulator of the renin-angiotensin system (RAS), a hormone system that regulates blood pressure, fluid and electrolyte balance, as well as systemic vascular resistance (for example, if RAS is abnormally active, blood pressure will be too high). Regarding the involvement of vitamin D in ACE2 and RAS modulation, adequate vitamin D supplementation is required to reduce RAS activity and increase soluble ACE2 (sACE2). This is remarkable because sACE2 retains its enzymatic activity becoming a target of the S-protein of SARS-CoV-2 (the spike (S) protein is found in the virus coat acting as the “key” to infect and enter into the cells coupling ACE2 receptors). So high levels of sACE2 may help to “disable” viral S-protein by joining them, promoting that S-protein loses its ability to bind to non-soluble membrane attached ACE2 receptors, preventing cells from being infected 14.
In summary, although the involvement of vitamin D in the immune system response has been widely demonstrated, conclusive evidence regarding the role of vitamin D in preventing or mitigating the severe respiratory complications of COVID-19 is lacking. Clinical evidence on vitamin D supplementation in patients with COVID-19 remains inconclusive, perhaps due to varied study end-points, variations in epidemiological characteristics (e.g., race and dietary habits) and different clinical settings. However, it is cost-effective to give vitamin D to boost immunity from prevention in COVID-19 era. Then, more randomized studies among different populations to determine variations in vitamin D requirements must be conducted.
References
- Mercola J, B Grant WB, Wagner CL. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients. 2020; 12: 3361. PMID: PMC7692080 DOI: 10,3390/nu12113361 ↩
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 10, 1373–1380. PMID: 32605780 DOI: 10.1016/j.jiph.2020.06.021 ↩
- Peng MY, Liu WC, Zheng JQ, Lu CH, Hou YC, Zheng CM, Song JY, Lu KC, Chao YC. Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D. Int. J. Mol. Sci. 2021, 22, 5251. PMID: 34065735DOI: 10.3390/ijms22105251 ↩
- Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 845–848. PMID: 32555424 DOI: 10.1038/s41591-020-0965-6 ↩
- Gwyer Findlay E, Currie SM, Davidson DJ. Cationic host defence peptides: Potential as antiviral therapeutics. BioDrugs 2013, 27, 479–493. PMID: 23649937 DOI: 10.1007/s40259-013-0039-0 ↩
- Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012, 8, e1002689. PMID: 22589721 DOI: 10.1381/journal.ppat.1002689 ↩
- Tian Y, Wang ML, Zhao J. Crosstalk between Autophagy and Type I Interferon Responses in Innate Antiviral Immunity. Viruses 2019, 11, 132. PMID: 30717138 DOI: 10,3390/v11020132 ↩
- Balla M, Merugu GP, Konala VM, Sangani V, Kondakindi H, Pokal M, Gayam V, Adapa S, Naramala S, Malayala SV. Back to basics: Review on vitamin D and respiratory viral infections including COVID-19. J. Community Hosp. Intern. Med.Perspect. 2020, 10, 529–536. PMID: 33194123 DOI: 10.1080/200096662020.1811074 ↩
- Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J. Biol. Chem. 2013, 288, 19450–19458. PMID: 23671281 DOI: 10.1074/jbc.M113.467670 ↩
- Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active 81.10.7090form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. PMID: 18981129 DOI:10.4049/jimmunol.181.10.7090 ↩
- Liu WC, Zheng, C.M.; Lu, C.L.; Lin, Y.F.; Shyu, J.F.; Wu, C.C.; Lu, K.C. Vitamin D and immune function in chronic kidney disease. Clin. Chim. Acta 2015, 450, 135–144. PMID: 26291576 DOI: 10.1016/j.cca.2015.08.011 ↩
- Nanzer AM, Chambers ES, Ryanna K, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013;132(2):297‐304.e3. PMID: 23683514 DOI: 1,.1,16/j.jaci.2013.03.037 ↩
- Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active 81.10.7090form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. PMID: 18981129 DOI:10.4049/jimmunol.181.10.7090 ↩
- Bian J, Li Z. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm Sin B. 2021; 11(1):1-12. PMID: 33072500 DOI:10.1016/j.apsb.2020.10.006 ↩