How exosomes can promote leukemia generation and growth
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults. This cancer is characterized by the rapid growth of immature myeloid precursors that occupy and alter the bone marrow (BM) niche where normal hematopoietic stems cells (HSCs) reside (the “niche” is the in vivo microenvironment where stem cells reside and receive stimuli that determine their regulation and fate). Current treatments include chemotherapy, radiotherapy and stem cell transplant; however, more knowledge is needed to provide concomitant treatment to improve the outcome of this malignancy.
Recent research has been focused to decipher the role of intercellular communication due to how cancer cells may corrupt its surrounding microenvironment. Exosomes are small vesicles that are secreted by a wide variety of normal and malignant 1 cells. These vesicles are recognized as key mediators of cell-to-cell communication 23. It has been shown that cancer-derived exosomes are capable of supporting cancer growth and disrupting the equilibrium of healthy tissues 4. However, the role of the exosomes secreted by AML cells remains to be elucidated. In this regard, Kumar B and collaborators demonstrate how AML-derived exosomes are involved in the transformation of the BM niche into a microenvironment that allow in one hand the leukemia growth and in the other hand to suppresses normal hematopoiesis 5.
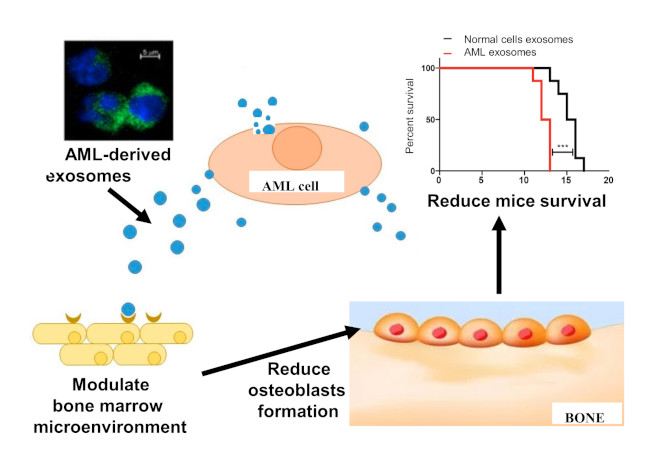
Figure modified from Bernardi S, Farina M. Exosomes and Extracellular Vesicles in Myeloid Neoplasia: The Multiple and Complex Roles Played by These “Magic Bullets” Biology 2021; 10: 105
First of all, Kumar B. et al. analyzed how AML cells affected the bone marrow microenvironment. Then, they injected AML cells into immunosuppressed mice and checked the different compartments of the BM niche after AML generation using FACS technology (a machine that using laser technology and controlled fluid flow is able to separate from a mix of a heterogenous cell population single cells in minuscule drops). FACS analysis revealed a decrease in the osteoblast population (the cells responsible for new bone formation) that is correlated with the loss or thinning of the osteoblast layer in AML sick animals.
Next, to test if AML cells remodel the BM microenvironment partly through exosome secretion, authors extracted exosomes from AML patients and labelled them with a fluorescent dye to analyze their localization. Then, they injected the labelled exosomes into mice and showed that internalized exosomes were found mainly in the BM stromal fraction but few exosomes were internalized in the hematopoietic fraction. Having determined that AML-derived exosomes were localized in the BM compartment, authors wanted to check if these exosomes were the responsible for the functional changes that occurs in the BM niche. Interestingly, they observed that animals that received AML-derived exosomes showed changes similar to those observed in mice transplanted with AML cells, including a decrease of primitive osteoblasts. According to this, they demonstrated loss of trabecular and cortical bone in the femurs of mice treated with AML-derived exosomes.
To understand how AML cells and AML-derived exosomes reduced osteoblasts formation in the BM niche, authors performed in vitro lineage differentiation assays. Firstly, they extract stem/progenitor cells (SPCs, cells that under specific culture conditions exhibit capability to proliferate and differentiate into specific terminal cells) from AML sick mice and maintained them in culture under osteogenic conditions (to promote their differentiation to osteoblats). Then, they observed that these cells exhibited reduced osteoblast formation compared with SPCs isolated from control mice. To assess whether AML-derived exosomes have a similar impact on differentiation of stromal progenitors, they treated SPCs coming from healthy mice with AML-derived exosomes or exosomes coming from control mice. Treated cells were divided and cultured in osteogenic (to promote osteoblast formation, which are cells responsible for bone formation), adipogenic (to promote adipocytes formation, cells responsible to store fatty acids) or chondrogenic (to promotes condrocytes formation, which are the cells responsible for cartilage formation) conditions. Interestingly, the results indicated that SPCs treated with AML-derived exosomes acquired an increased ability to differentiate into adipocytes, but a decreased ability to differentiate into osteoblasts and chondrocytes, compared with SPCs treated with exosomes from control mice.
To determine whether AML-derived exosomes functionally altered the BM niche’s ability to support leukemia generation, authors asked whether preconditioning the BM niche using AML-derived exosomes could facilitate AML growth. Then, mice pretreated with weekly doses of AML-derived exosomes were then injected with AML cells and analyzed for leukemia generation. These mice showed a significantly higher rate of leukemia cell implantation and a shorter survival than controls pretreated with weekly doses of exosomes derived from control mice. Finally, to test whether AML-derived exosomes are required for leukemia growth, authors reduced exosome secretion in AML cells silencing the expression of a protein implicated in the exosome release process 6. When they injected these cells into mice, they showed that mice that received the silenced cells survived longer than mice that received control AML cells, indicating that reduce exosome secretion delay AML growth. According to the present data, it has been previously shown that osteoblast elimination converts bone marrow into a proliferation-promoting environment for malignant stem cells 7 and accelerates leukemia development 8.
Considering these results, novel therapeutic strategies could be developed to fight AML. Disrupting exosome production and/or secretion in AML cells or directly targeting factors induced by AML-derived exosomes may provide new avenues to prevent AML-induced transformations of the BM niche.
References
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10: 1470-1476. PMID: 19011622 DOI: 10.1038/ncb1800 ↩
- Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 2014; 29: 116-125.PMID: 24959705 DOI: 10.1016/j.ceb.2014.05.004 ↩
- Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol 2011; 33: 441-454. PMID: 21688197 DOI: 10.1007/s00281-010-0234-8 ↩
- Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer–the emerging science of cellular ‘debris’. Semin Immunopathol 2011; 33: 455-467. PMID: 21318413 DOI: 10.1007/s00281011-0250-3 ↩
- Kumar B, Garcia M, Weng L, Jung X, Murakami JL et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia 2018; 32: 575-587. PMID: 28816238 DOI: 10.1038/leu.2017.259 ↩
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12: 19-30. PMID: 19966785 DOI: 10.1038/ncb2000 ↩
- Zhao M, Li L. Osteoblast ablation burns out functional stem cells, Blood 2015; 125: 2590-2591. 2015 Apr 23;125(17):2590-1. PMID: 25907901 DOI: 10.1182/blood-2015-03-633651. ↩
- Bowers M, Zhang B, Ho Y, Agarwal P, Chen CC, Bhatia R. Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development. Blood 2015; 125: 2678-2688. PMID: 25742698 DOI: 10.1182/blood-2014-06-582924 ↩