Exosomes in cancer: corrupted messages that transmit the metastatic impulse
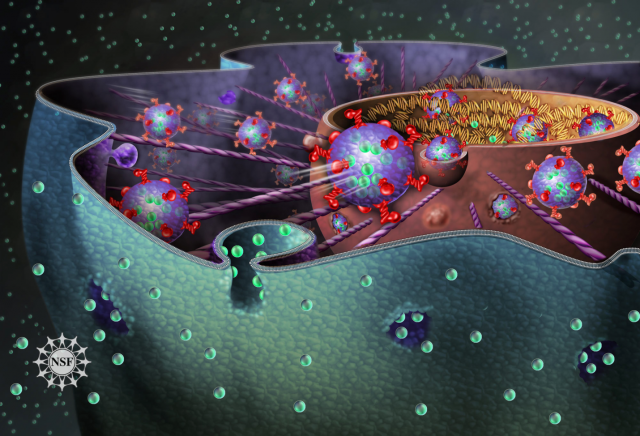
Just as people constantly exchange messages via email, cells communicate with each other and their microenvironment by sending and receiving packages of information; and as we react in one way or another depending on the news we get, cells respond to the “message” by changing the expression of their genes. Following the analogy, in a similar way as our computers get corrupted when opening malicious emails, cells can turn malignant when receiving packages that are delivered by cancer cells. Beware of spam!
Exosomes were described back in the 1980´s as “exfoliated membrane vesicles that could have a physiologic function” 1. Indeed, these membrane vesicular bodies resulting from the fusion of late endosomes with the inner face of the plasma membrane were thought to be trash bags that normal cells sent out carrying unnecessary molecules that had not been degraded by the lysosomal system. However, what those pioneering studies did not suspect, is that these vesicles are in fact shuttles working as highly specialized functional extensions of the cells that serve as systemic communication channels (also reviewed by Carlos Romá-Mateo in Mapping Ignorance)
Virtually all cell types in the organism release exosomes containing information in the form of nucleic acids -RNA or DNA molecules- and proteins that are assimilated by recipient cells at distant locations (Figure 1). For example, platelets, the cells responsible for clogging injured blood vessels, emit exosomes to regulate the coagulation process; immune cells integrate antigen-containing exosomes to initiate an immune response, or generate exosomes to modulate it; and these vesicles are also mediators of crosstalk between the fetus and the mother during pregnancy.
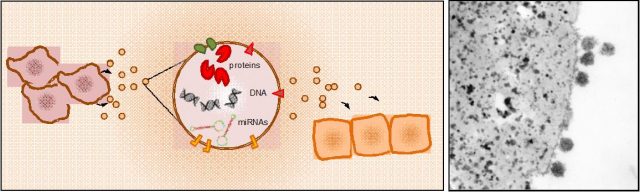
Cancer cells, however, seem also to have taken advantage of this mean of communication to increase their area of action. What´s more, a recent report has shown that these tiny vesicles not only carry the message, but are also equipped with the non-coding RNA-processing machinery that produce mature RNA molecules able to alter the transcriptome of recipient cells 2. These biopackages of horizontal information transference are also used by cancer cells to facilitate the establishment of metastases. The group of David Lyden, described in 2012 how exosomes secreted by melanoma cells promoted the metastatic colonization in the lung. By analyzing the cargo of tumor cell-derived exosomes, authors found high levels of pro-oncogenic proteins that were delivered through the bloodstream and induced vascular permeability in the lung parenchyma. In parallel, these vesicles re-educated bone-marrow derived cells -progenitor cells that maintain tissue regeneration and organ vascularization-, to generate a pro-metastatic microenvironment hospitable for disseminating tumor cells 3. Importantly, circulating exosomes containing oncoproteins were much more abundant in patients with worse prognosis, indicating that they could constitute biomarkers to predict the appearance of metastasis.
Another work by the same group has recently described how exosomes release is enhanced in pancreatic adenocarcinoma cells (PDAC) in association with the generation of liver metastases 4. The protein load confined within PDAC-derived exosomes was preferentially assimilated by Kupffer cells (KC), the resident liver macrophages, and contained high levels of pro-inflammatory cytokines. Authors showed how these molecules orchestrated the sequential cascade that acclimatized the liver parenchyma for invading cancer cells. Activated KC resulting from exosome uptake showed a marked over-expression of TGFβ signaling molecules that prepared the liver environment for the arrival of metastatic cells, by inducing liver fibrosis and macrophage infiltration. As such, authors demonstrated that impairment of fibronectin deposition –that abrogated liver fibrosis-, ablation of macrophage recruitment, or depletion of the cytokines enclosed on PDAC-derived exosomes, inhibited the ability of KC to induce TGFβ signaling and avoided the formation of the liver pre-metastatic niche. Strikingly, these corrupted exosomes isolated from the circulation were detected on mice with pre-invasive pancreatic lesions (PanIN), suggesting that metastatic dissemination is forethought long before becoming an invasive carcinoma. Likewise, the presence of high levels of malignant circulating exosomes in PDAC patients was associated with the presence of liver metastases as compared with patients that had not shown disease progression.
Finally, a recently published article used intravital microscopy in mice to track the movement of exosomes produced by cancer cells, and study the behavior of assimilating neighboring cells 5. The study by Zomer and co-workers use a Cre-LoxP system to induce a color switch on cells that take up exosomes from metastatic cells expressing the Cre recombinase (Figure 2). Once neighboring cells take up Cre- (and additional protein and nucleic acids coming from metastatic cells)-containing exosomes, they activate the GFP cassette (green-fluorescent protein), allowing investigators to follow their activity in vivo.
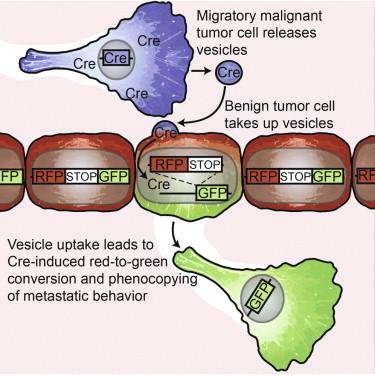
Through this elegant approach, authors could record in vivo how the microvesicles released from metastatic cancer cells were incorporated not only by normal cells -macrophages and neutrophils-, but also by non-metastatic tumor cells that turned into highly invasive after taking up the biomolecules enclosed in the exosomes (Figure 2).
In this line, researchers are currently investigating the potential of exosome-based signatures as prognostic biomarkers to anticipate the appearance of metastasis. Information is power. Thus, if we are able to decipher the messages that cancer cells spread through the organism, we will be able to use it against them to predict their movements and design preventive treatments able to reduce cancer mortality.
References
- Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981 Jul 6; 645(1):63-70. ↩
- Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014 Nov 10; 26(5):707-21. ↩
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012 Jun; 18(6):883-91. ↩
- Costa-Silva B et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015 Jun; 17(6):816-26. DOI: 10.1038/ncb3169 ↩
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015 May 21; 161(5):1046-57. DOI: 10.1016/j.cell.2015.04.042 ↩