Mechanism of brain cell migration unravelled
Mechanism of brain cell migration unravelled
Author: José R. Pineda got his Ph.D. from University of Barcelona in 2006. Since 2007 he has worked for Institut Curie and The French Alternative Energies and Atomic Energy Commission. Currently, he is a researcher of the UPV/EHU. He investigates the role of stem cells in physiologic and pathologic conditions.
During brain development, immature neurons must migrate long distances through complex environments to reach their final destination. To guide themselves, they have to establish several interactions at molecular level between their membrane receptors and their environment. The work done by Akkermans and collaborators 1 is an international collaboration from Spain, France and United Kingdom laboratories published in “Cell”. They have identified how the neuronal receptor Unc5 and the molecule Glipican 3 (GPC3) collaborate and interact to guide brain cells in both healthy process such as the neuronal precursor migration during development that allows the formation of the brain as well as the pathogenic process such as the spread of a type of brain cancer called neuroblastoma.
This work is an example of fine-tuned characterization of cellular mechanism in which Akkermans and collaborators identifies the points where the two proteins connect, reveals the structure of the new Unc5-GPC3 complex and determines its key role in the migration of neurons and tumor cells. It also delves into the mechanisms that regulate cell migration in a very precise way, and notes how some molecular mechanisms that regulate cell migration are highly conserved between healthy and malign cells.
During the development of the cerebral cortex, immature neurons (cells that will become future neurons) derived from neuronal progenitors use the of stem cells (called radial glia) apical projections to climb and reach their final position. Thus, during migration, immature neurons must interact with these projections, although the exact mechanism and the involved proteins responsible for the interaction were poorly understood.
The discovery of the mechanisms of the molecular guidance of brain cells was possible thanks to the structural analyses through crystal structures of the protein complex. Using an unbiased enzyme-linked immunosorbent assay, they identified the binding sites between the proteins, finding that GPC3 was a ligand of Unc5D and using surface plasmon resonance binding experiments confirming the biomolecuar interaction forming an octameric complex. They wondered to elucidate if there was a particular “cis” or “trans” conformation to interact the proteins, finding that cis-interaction silenced trans binding. To generate additional tools for the functional characterization of the interaction, they used two nanobodies (the heavy-chain of antibodies), which bind to murine and human GPC3 core and realized pull-down experiments. Because Unc5 receptors were known to induce repulsive signalling in neuronal cell guidance, they assessed if GPC3-Unc5 interaction mediated contact-repulsion. They plated on alternating stripes containing purified GPC3core and the mutant GPC3coreUG (that had lost the affinity for Unc5). In these assays, cortical neurons preferentially migrated on the mutant protein stripes, demonstrating that GPC3core elicits a repulsive effect via an Unc5-dependent mechanism. However, when cells were given the choice between GPC3core (wild type or mutant) and nanobody, the neurons were strongly repelled by both WT and the mutant protein. These results suggest that unknown additional GPC3-receptors were able to cause repulsion independently of the Unc5-GPC3interaction. In light of these results they tested in stripe assays other different cell types such as cancer cells like HeLa, N2A, and SY5Y, reproducing, as previously demonstrated in neurons, that these cells preferred to grow on mutant. These results concluded that GPC3-Unc5 interaction mediates contact-repulsion, which may contribute to cellular migration.
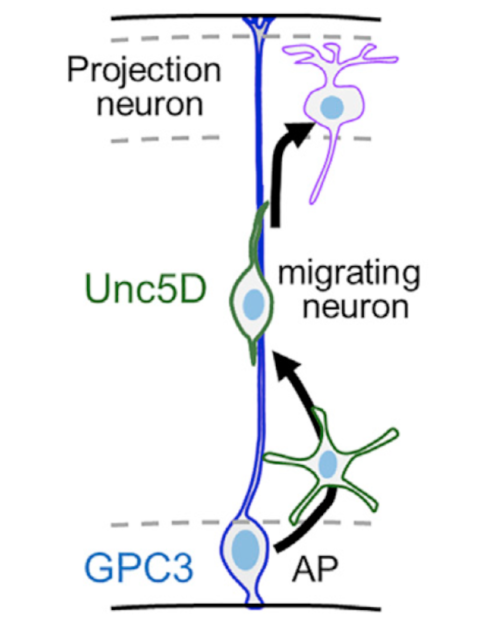
At this point, they validated the mechanism in vivo. They found that GPC3 was expressed in the germinal zone and Unc5D in migrating neurons in the developing mouse brain cortex (see figure). They highlighted the importance of the migration of the main neurons of the cerebral cortex (this is a decisive process for the correct formation of the neural circuits responsible of cognitive functions such as language, cognition, abstract thinking, among others). Moreover, they demonstrated using in-utero electroporation that GPC3-Unc5 interaction is required for radial neuronal migration in vivo. Complementary to this, using data from 16 different neuroblastoma patient samples and analyzing the expression of GPC3 and Unc5 of single cell RNA-seq they also demonstrated that GPC3-Unc5 interaction is required for neuroblastoma cell migration in vivo and that endothelial cells highly expressed Unc5 receptors. Focusing at molecular level, using SY5Y neuroblastoma cell line, modulating its levels of GPC3 by siRNA-transfection and testing the cells in transwell invasion assay they found that GPC3 siRNA cells migrated less compared to mock-transfected cells. Engrafting SY5Y cells into an avian pre-migratory trunk of neural crest, they observed the pattern of migration following a stereotypical ventral migratory path to the developing sympathetic ganglia and adrenal medulla. Tumour formed tumor masses before undergoing secondary metastatic-like dissemination. Compared to embryos engrafted with scramble siRNA-transfected SY5Y, GPC3 siRNA-transfected cells formed tumor masses almost exclusively outside the proximal and distal sympatho-adrenal (SA) territories. This demonstrated that interfering with the neuroblastoma source of GPC3 disrupts cell migration and targeting to the primary tumor site, and Unc5-GPC3 interaction directs neuroblastoma collective migration.
Last but not least, the team found that the Unc5-GPC3 complex is present in other regions of the brain and, consequently, other neurons could also use this mechanism to migrate.
Until now, cells were thought to use different mechanisms to migrate in completely independent biological environments. On the contrary, the new results point out that the guidance mechanisms during cell migration can be shared and reused by different cell types. For example, the cerebral cortex also receives other neurons – known as interneurons – that also express proteins from this complex. Therefore, it will be very interesting to check the functions of this complex in other neuronal types in future studies.
Overall, the knowledge and tools generated in this work can be applied in very different contexts, such as the migration of other cell types, or the application of new strategies to control this process in certain pathologies, such as brain cancer. It should be remembered that in the case of certain tumors, such as neuroblastoma, the expression of the Unc5 protein is very high. For this reason, it is possible to speculate that this factor could be implied in the migration and dissemination of this intracerebral tumor.
References
- Akkermans O, Delloye-Bourgeois C, Peregrina C, Carrasquero-Ordaz M, Kokolaki M, Berbeira-Santana M, Chavent M, Reynaud F, Raj R, Agirre J, Aksu M, White ES, Lowe E, Ben Amar D, Zaballa S, Huo J, Pakos I, McCubbin PTN, Comoletti D, Owens RJ, Robinson CV, Castellani V, Del Toro D, Seiradake E. (2022) GPC3-Unc5D complex structure and role in cell migration Cell doi:10.1016/j.cell.2022.09.025 ↩