An ancient stress-related mechanism helps worms keep their cool
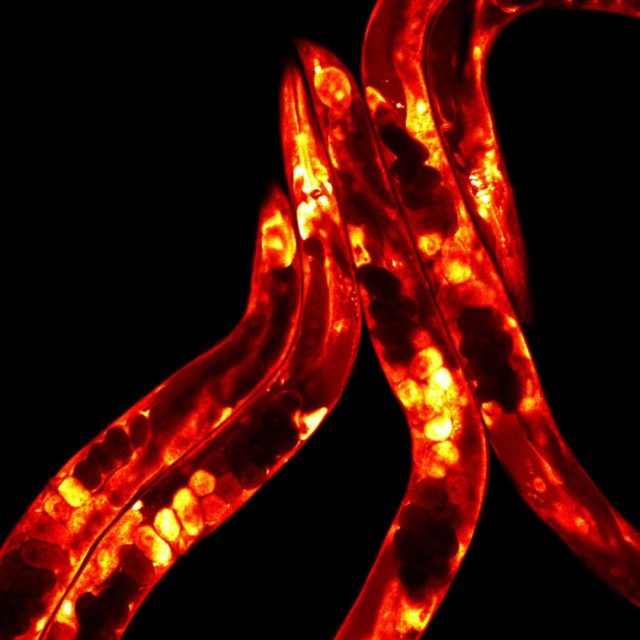
How an organism can sense and cope with warming temperatures is key for survival, particularly under current global warming trends. The importance of this subject was recognized by 2021’s Nobel prize in Physiology & Medicine awarded to work on TRP channels, key mammalian peripheral heat sensors.
Endotherms -such as mammals and birds – maintain their body temperature through skin-based TRP channels that relay temperature information to a region of the brain called the hypothalamus, where specific neurons act as a thermostat to regulate body temperature.
C. elegans as a model for ectotherm temperature regulation
In contrast, ectotherms – such as reptiles, fish, and invertebrates – cannot control their own body temperature. In a recently published article in PLOS Biology 1, Laetitia Chauve and colleagues took advantage of the transparent worm C. elegans to show that although ectotherms cannot internally regulate their own body temperature like mammals, they still centrally coordinate their response to heat.
Thanks to its transparency, they could visualize fat metabolism by fluorescent reporters, since fat metabolism is closely related to temperature adaptation in these animals. Using these tools, they turned off and on the function of a specific gene in neurons, heat shock factor 1(HSF-1), the master regulator of the stress responses, finding that it had a striking effect on fat metabolism in the gut.
The heat shock response is conserved in all animals, including bacteria. When exposed to heat stress, cells activate HSF-1, which rapidly induces the expression of heat shock proteins involved in chaperoning and refolding heat-damaged proteins. Furthermore, HSF-1 is recognized as a pro-longevity factor because it clears aggregates and misfolded proteins from old cells.
Interestingly, although worms lack sophisticated TRP channels to sense heat in their epidermis/skin, they have cleverly co-opted this ancient system that responds to heat, to allow for the detection of increasing temperatures. HSF-1 was found to be necessary and sufficient to coordinate a complex neuro-hormonal response, mediated – at least in part – by a secreted Bone Morphogenetic Protein (BMP), which signals from neurons into the gut, so that under warm conditions saturated fatty acids are produced, allowing worms to survive and live longer under warm conditions.
In summary, this is the first study to report that ectotherms co-opted ancient heat sensing cellular responses as a thermostat to centrally coordinate complex adaptive responses to warming. Further work should investigate if a similar role is found in mammals, which can be relevant in the context of ageing and obesity.
References
- Chauve L, Hodge F, Murdoch S, Masoudzadeh F, Mann H-J, Lopez-Clavijo AF, et al. (2021) Neuronal HSF-1 coordinates the propagation of fat desaturation across tissues to enable adaptation to high temperatures in C. elegans. PLoS Biol 19(11): e3001431. doi: 10.1371/journal.pbio.3001431 ↩