Losing genes can be good, hummingbirds are a nice example
Author: Ramón Muñoz-Chápuli has been Professor of Animal Biology in the University of Málaga until his retirement. He has investigated for forty years in the fields of developmental biology and animal evolution.
Hummingbirds are fascinating animals. They are the only birds able to true hovering and backward flight. This ability allows them to collect nectar from flowers that have coevolved with hummingbirds and are pollinated by them. Hovering requires complex musculoskeletal adaptations but also significant specializations of the metabolism. Hummingbirds have the highest mass-specific metabolic rate measured in vertebrates, twice the maximum recorded in mammals and ten times higher than human athletes. Wingbeat frequency during hovering is between 30 and 60 Hz. The hummingbird heart, twice as large as predicted by comparison with other birds, reaches 1400 beats per minute, and the cardiac output (blood volume ejected by the heart in a minute) equals five times the body weight 12. These performances may be at the upper limits of what is structurally and functionally possible in vertebrates 3.
Thus, hummingbirds require a continuous supply of food to fuel this vast energetic demand. Sucrose-rich nectar is the preferred source of energy for hummingbirds. The abundant intestinal sucrase splits sucrose in glucose and fructose that are rapidly absorbed by the intestinal epithelium and also through a paracellular pathway 4. These monosaccharides are directly transported by the blood to the muscle. Differently to what happens in mammals, hovering hummingbirds are able to utilize fructose and glucose equally as a fuel 5. Additionally, the hummingbird muscles show a very high capillary density, and 35% of the volume of their fibres is occupied by mitochondria.
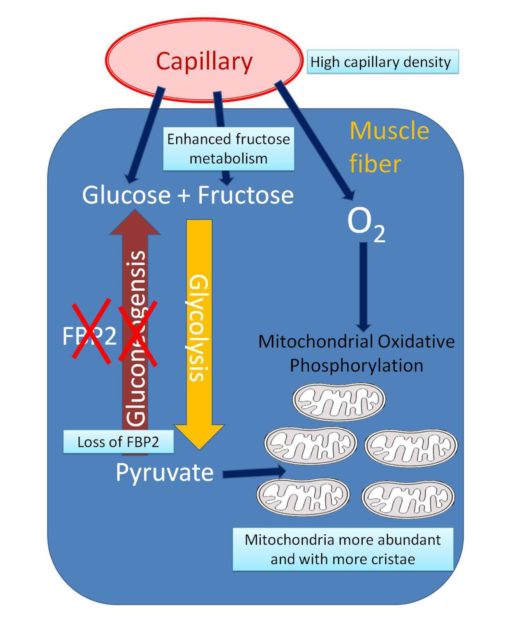
All these physiological adaptations allow hummingbirds an exceptional flight performance. However, a surprising genetic adaptation, based on a gene inactivation, has been recently described 6. The study of the genome of the long-tailed hermit (Phaethornis superciliosus) together with two previously sequenced hummingbird genomes, have shown inactivating mutations in the FBP2 gene, coding for the muscle-specific fructose-bisphosphatase 2 enzyme. This inactivation is not compensated by expression of the liver and kidney specific isoform of this enzyme, FBP1. Thus, there is no fructose bisphosphatase enzymatic activity in the hummingbird muscle, in contrast to other birds and vertebrates.

FBP2 catalyzes a rate-limiting step in gluconeogenesis, the synthesis of glucose from pyruvate. Many steps of this metabolic pathway are the opposite to those found the glycolysis pathway, the conversion of glucose into pyruvate. In aerobic conditions, pyruvate supplies energy to cells through the Krebs cycle, a chain of reactions occurring in the mitochondria. To check the consequences of this gene loss, the authors of the study inactivated FBP2 in cultured quail myoblast cells using a CRISPR-Cas9 system. The FBP2-deficient myoblasts showed upregulation of glycolysis, mitochondrial respiration and mitochondrial number, in a similar way to that observed in hummingbird muscle.
The transcriptome of the hummingbird muscle showed downregulation of genes involved in fatty acid and amino acid metabolism, while the genes related with carbohydrate metabolism, the Krebs cycle, the electron transport chain function, and the formation of inner mitochondrial membrane folds were upregulated.
The loss of FBP2 in hummingbird muscle shows striking similarities to some human cancers which downregulate fructose bisphosphatases enhancing the glycolytic pathway. This downregulation contributes to the Warburg effect, an increase of the glucose uptake and preferential use of glycolysis to generate lactate for rapid growth. In fact, forced expression of FBP2 in sarcoma cell inhibits tumor growth by antagonizing elevated glycolysis and restraining mitochondrial biogenesis and respiration 7.
In summary, the loss of the FBP2 function, through inactivating mutations of the gene in hummingbird muscle, increased the metabolic potential for energy production from dietary sugars. This gene loss was probably essential for the evolution of the hovering flight and highlights the relevance that gene loss can have for adaptation.
More on the subject:
Losing genes can be a win in evolutionary terms
References
- Suarez RK, Lighton JR, Brown GS, Mathieu-Costello O. Mitochondrial respiration in hummingbird flight muscles. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4870-3. doi: 10.1073/pnas.88.11.4870. ↩
- Suarez RK, Welch KC. Sugar Metabolism in Hummingbirds and Nectar Bats. Nutrients. 2017 Jul 12;9(7):743. doi: 10.3390/nu9070743. ↩
- Suarez RK. Hummingbird flight: sustaining the highest mass-specific metabolic rates among vertebrates. Experientia. 1992 Jun 15;48(6):565-70. doi: 10.1007/BF01920240. ↩
- McWhorter TJ, Bakken BH, Karasov WH, del Rio CM. Hummingbirds rely on both paracellular and carrier-mediated intestinal glucose absorption to fuel high metabolism. Biol Lett. 2006 Mar 22;2(1):131-4. doi: 10.1098/rsbl.2005.0388. ↩
- Chen, C.C.W.; Welch, K.C., Jr. Hummingbirds can fuel expensive hovering flight completely with either exogenous glucose or fructose. Funct. Ecol. 2014, 28, 589–600. ↩
- Osipova E, Barsacchi R, Brown T, Sadanandan K, Gaede AH, Monte A, Jarrells J, Moebius C, Pippel M, Altshuler DL, Winkler S, Bickle M, Baldwin MW, Hiller M. Loss of a gluconeogenic muscle enzyme contributed to adaptive metabolic traits in hummingbirds. Science. 2023 Jan 13;379(6628):185-190. doi: 10.1126/science.abn7050. ↩
- Huangyang P, Li F, Lee P, Nissim I, Weljie AM, Mancuso A, Li B, Keith B, Yoon SS, Simon MC. Fructose-1,6-Bisphosphatase 2 Inhibits Sarcoma Progression by Restraining Mitochondrial Biogenesis. Cell Metab. 2020 Jan 7;31(1):174-188.e7. doi: 10.1016/j.cmet.2019.10.012. ↩