Bio-mining pyrite with no oxygen
Bio-mining pyrite with no oxygen
Pyrite, also known as “fool’s gold,” is an abundant iron sulphide mineral in the Earth’s crust. All organisms need iron and sulphur to grow. Because pyrite does not dissolve in oxygen-free conditions, scientists previously thought that organisms could not use pyrite in the absence of oxygen. Research shows that certain single-celled microorganisms can dissolve pyrite in the absence of oxygen. These microorganisms mine iron and sulphur from the pyrite to build biocatalysts needed for growth.

A longstanding paradigm is that pyrite (FeS2) is stable at low temperature and unavailable to microorganisms in the absence of oxygen and oxidative weathering. Common mining practices extract the mineral and expose it to oxygen to leach and concentrate iron and other metals. This process can generate extremely acidic and toxic run-off, commonly referred to as acid-mine drainage.
Scientists show 1 that methanogens can dissolve pyrite without oxygen at low temperature and utilize dissolved products to meet the organism’s nutritional requirements for iron and sulphur. And that they can do so without generating acid.
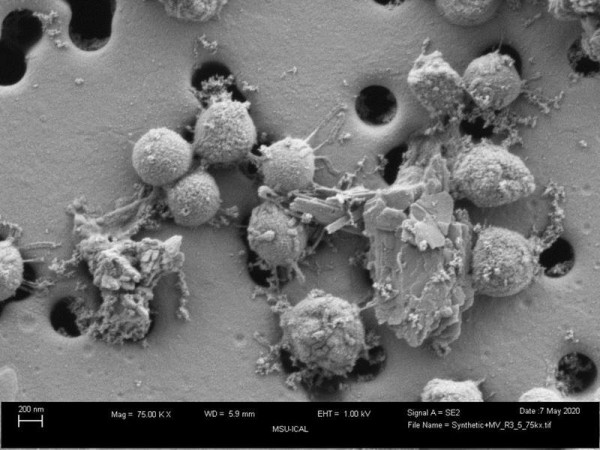
Microscopic imaging and growth experiments show that cells require direct access to pyrite to dissolve the mineral and/or to assimilate iron sulphide that forms on the mineral surface. The iron content of cells grown with pyrite is significantly higher than cells grown with other iron sources, with excess iron stored intracellularly in nanoparticles. Cells that bio-mine pyrite hyperaccumulate iron, meaning they have higher concentrations of iron than the surrounding soil. This ability highlights their potential application for extracting iron and other elements from pyritic ores.
These findings demonstrate that pyrite is bioavailable to anaerobic methanogens and can be mobilized in low-temperature, anoxic environments. Given that methanogens evolved at least 3.46 billion years ago, these data indicate that the microbial contribution to the iron and sulphur cycles in ancient and contemporary anoxic environments may be more complex and robust than previously recognized, with potential impacts on numerous biogeochemical cycles.
These observations also highlight the ability of methanogens to transform iron sulphide minerals abundant on Earth into biocatalysts of biotechnological importance. This includes those that generate biofuels (hydrogen and methane) and fertilizer (ammonia).
Related:
Earth’s oxygen has varied dramatically over time – here’s how new data could help us spot alien life
References
- Payne, D., Spietz, R.L. & Boyd, E.S. (2021) Reductive dissolution of pyrite by methanogenic archaea. ISME J 15, 3498–3507. doi: 10.1038/s41396-021-01028-3 ↩