Venetoclax therapies in acute myeloid leukemia
Venetoclax therapies in acute myeloid leukemia
Acute myeloid leukemia (AML) is a disease of older persons that has a dismal outcome and patients unfit for intensive chemotherapy exhibit a median survival of only 5 to 10 months 1. However, the therapeutic landscape of AML is changing with the appearance of new-targeted drugs such as BH3 mimetics that inhibit prosurvival BCL2 family proteins 2. In AML, the activity of venetoclax as a single agent has been modest 3; nevertheless, combinations with either hypomethylating agents or low-dose chemotherapy demonstrated impressive clinical benefit in older/unfit patients 4. However, despite promising results of phase III clinical trials, longer follow-up has shown that venetoclax combinations extend median overall survival (OS) by only 4 to 5 months 5. Thus, these data highlight the critical need for more effective venetoclax combination therapies.
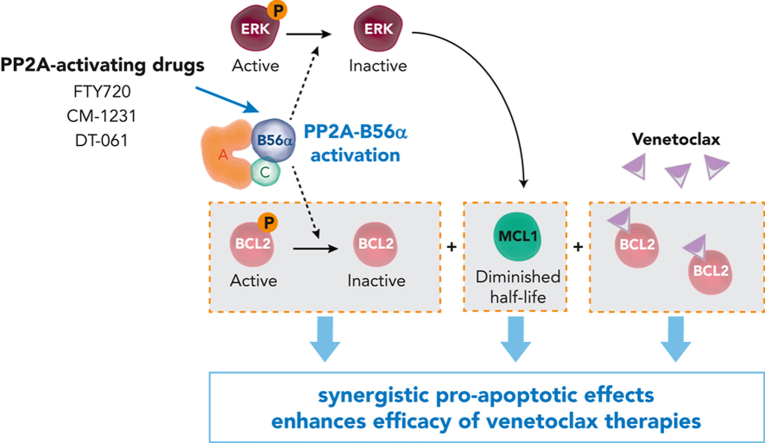
Protein phosphatase 2A (PP2A), one of the main serine (Ser)/threonine (Thr) phosphatases, is a tumor suppressor that regulates essential functions 6. The substrate specificity is achieved by the formation of distinct heterotrimeric holoenzymes, each containing a catalytic (C), a scaffold (A), and a regulatory (B) subunit. Regulatory B subunits are critical because they dictate substrate specificity and localization of PP2A complexes. In AML, it has been reported inactivation of PP2A in ~70% of cases (7) and that pharmacological restoration of PP2A activity has potent antitumor effects 7. Given that PP2A promotes cancer cell apoptosis by affecting the phosphorylation state of different proteins in the mitochondrial apoptotic pathway (6), Peris I and collaborators hypothesized that PP2A reactivation might have therapeutic value in AML by triggering mitochondrial apoptosis. Authors show that PP2A-activating drugs sensitize AML cells to venetoclax-mediated cytotoxicity. Of importance, they demonstrate that the antileukemic effect of clinically approved venetoclax-azacitidine combination is synergistically enhanced by PP2A reactivation in vitro, in primary AML blasts, and in vivo 8.
Firstly, to investigate whether the PP2A-activating compound FTY720 enhances venetoclax activity, they first evaluated the cytotoxic effect of both drugs in 14 human AML cell lines. Cell viability assays after FTY720, venetoclax, or the combined treatment at different concentrations showed that FTY720 plus venetoclax resulted in synergistic antileukemic effects in 10 of the 14 AML cell lines tested. They also evaluated the effect of these drugs in human AML xenografts in zebrafish embryos 9. As cellular models, they chose 4 well-characterized AML cell lines with different genetic/cytogenetic backgrounds. Compared with the control and single-therapy groups, treatment with FTY720 plus venetoclax synergistically reduced cell proliferation and colonization in all tested AML zebrafish models.
To investigate how FTY720 synergizes with venetoclax, they carried out time-course analyses of cell death through annexin V/propidium iodide staining and caspase-3 and -7 activation in selected AML cell lines. Thus, they observed that combination therapy increased the number of apoptotic cells (apoptotic cells are cells that activates their suicide program), compared with single treatments, and induced a synergistic time-dependent activation of caspase-3 and -7. Next, they measured the changes in the expression of pro-apoptotic and anti-apoptotic proteins of the BCL2 family under drug treatment and they detected marked reduction in MCL1 protein levels upon addition of venetoclax with FTY720 treatment. Since literature has suggested a role for PP2A in the regulation of BCL2, they checked for changes in BCL2 phosphorylation at Ser70 10 and interestingly they observed that FTY720 and venetoclax treatments synergistically decreased BCL2 phosphorylation at this residue. This data suggests that PP2A activation is responsible for MCL1 and p-BCL2 modulation, and that these effects are significantly potentiated by venetoclax. Given that extracellular signal-regulated kinase (ERK)-mediated phosphorylation of MCL1 at Thr163 delay MCL1 degradation 11 and that ERK is a target of PP2A 12, they hypothesized that PP2A activation could modulate ERK-driven MCL1 protein stability in AML cells. According with these publications, they observed that FTY720 in combination with venetoclax resulted in a significant reduction in ERK phosphorylation and of MCL1 at Thr163. Thus, to evaluate whether ERK inactivation by FTY720-venetoclax combination therapy is important for its efficacy, AML cells were treated with combined trametinib (MEK-ERK inhibitor) and venetoclax. As FTY720-venetoclax, this combination had anti-leukemic effects and showed decreased MCL1 protein levels.
Next, they analyzed the anti-leukemic activity of FTY720 and venetoclax in 5 primary AML samples. After performing colony-forming unit (CFU) assays they observed that the response to this combined treatment was synergistic in 4 of the 5 samples. To extend these results, they collected leukemic cells from an additional 21 patients with AML at the moment of diagnosis. Accordingly to AML cell lines, the combination of the treatment with FTY720 plus venetoclax induced synergistic changes in cell viability in 13 of the 21 primary AML samples. To confirm the underlying mechanism of the combined therapy, they performed drug treatment and then, they did western blot analyses of the different AML samples to check the phosphorylations. Consistent with the observations in cell lines, levels of p-BCL2, p-ERK, and MCL1 were significantly decreased upon treatment with FTY720 and venetoclax.
PP2A-B56α and PP2A-B55α complexes regulate most of the key signal transduction pathways involved in AML development and progression (6). To investigate their respective roles in the observed synergistic responses, authors used CRISPR-Cas9 assays to generate sublines derived from the HL-60 AML cell line lacking B56α or B55α subunits. Using this technique, they showed that inactivation of B56α impeded changes in p-BCL2, p-ERK, and MCL1 protein levels following the combined therapy, thereby abolishing the synergistic effects. In contrast, B55α inactivation had no effect on either protein expression changes or cell viability, pointing to a critical role for the PP2A-B56α complex in modulating the cytotoxic effects observed with combination treatment. Accordingly, zebrafish models revealed that loss of B56α rendered HL-60 cells more resistant to the combination therapy in vivo, whereas inactivation of B55α did not change therapy responses. Moreover, in vivo treatments using immunodeficient NSG mice that underwent xenotransplantation with HL-60 wild-type B56α, showed a significant reduction in tumor volume in the combined treatment group, which was not observed in mice carrying B56α-inactivated HL-60 cells. Furthermore, p-BCL2, p-ERK, and MCL1 levels increased in HL-60 cells with B56α inactivation upon the combination treatment.
Venetoclax in combination with hypomethylating agents is the new standard treatment for older/unfit patients with AML (5). Based on their results, authors hypothesized that a triple therapy consisting of FTY720-venetoclax-azacitidine might drive better efficacy than venetoclax-azacitidine. The triple combination had greater synergistic effects than venetoclax plus azacitidine in AML cell lines and in zebrafish xenograft. Interestingly, only the triple therapy led to a significant reduction in the levels of p-BCL2, p-ERK, and MCL1 proteins. Next, they tested this triple-combination therapy on primary AML cells, which was also more effective than venetoclax plus azacitidine and induced changes in p-BCL2, p-ERK, and MCL1 expression.
In conclusion, their findings provide preclinical evidence for combining PP2A-activating drugs with venetoclax or venetoclax-azacitidine as a new therapeutic strategy for the treatment of AML.
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
References
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373:1136-52. PMID: 26376137 DOI: 10.1056/NEJMra1406184. ↩
- Diepstraten ST, Anderson MA, Czabotar PE, Lessene G, Strasser A, Kelly GL.The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat Rev Cancer. 2022;22:45-64. PMID: 34663943 DOI: 10.1038/s41568-021-00407-4. ↩
- Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016;6:1106-1117. PMID: 27520294 DOI: 10.1158/2159-8290.CD-16-0313. ↩
- DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, Arellano M, Frattini MG et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216-228. PMID: 29339097 DOI: 10.1016/S1470-2045(18)30010-X. ↩
- Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, Kim I, Stevens DA et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135:2137-2145. PMID: 32219442 DOI: 10.1182/blood.2020004856. ↩
- Arriazu E, Pippa R, Odero MD. Protein Phosphatase 2A as a Therapeutic Target in Acute Myeloid Leukemia. Front Oncol. 2016;6:78. PMID: 27092295 DOI: 10.3389/fonc.2016.00078. eCollection 2016. ↩
- Cristóbal I, Garcia-Orti L, Cirauqui C, Alonso MM, Calasanz MJ, Odero MD. PP2A impaired activity is a common event in acute myeloid leukemia and its activation by forskolin has a potent anti-leukemic effect. Leukemia. 2011;25:606-14. PMID: 21233840 DOI: 10.1038/leu.2010.294. ↩
- Peris I, Romero-Murillo S, Martínez-Balsalobre E, Farrington CC, Arriazu E, Marcotegui N, Jiménez-Muñoz M, Alburquerque-Prieto C et al. Activation of the PP2A-B56α heterocomplex synergizes with venetoclax therapies in AML through BCL2 and MCL1 modulation. Blood. 2023;141:1047-1059. PMID: 36455198 DOI: 10.1182/blood.2022016466. ↩
- MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat Rev Drug Discov. 2015;14:721-31. PMID: 26361349 DOI: 10.1038/nrd4627. ↩
- Ruvolo PP, Clark W, Mumby M, Gao F, May WS. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem. 2002;277:22847-52. PMID: 11929874 DOI: 10.1074/jbc.M201830200. ↩
- Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301-15. PMID: 15241487 DOI: 10.1038/sj.onc.1207692. ↩
- Letourneux C, Rocher G, Porteu F. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J. 2006;25:727-38. PMID: 16456541 DOI: 10.1038/sj.emboj.7600980. ↩