Antibody-drug conjugates for the treatment of acute myeloid leukemia
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Acute myeloid leukemia (AML) is the most common acute leukemia in adults and a common pediatric cancer. Most often, AML develops from cells that would turn into white blood cells. AML is divided into different subtypes based on the cell type and maturation stage. Currently, monocytic AML, including acute myelomonocytic leukemia (M4) and acute monocytic leukemia (M5), accounts for approximately 30% of all cases of AML. Despite actual treatments, AML relapse and most patients succumb within the 5 years from diagnosis. Patients with AML with a significant monocytic component are more likely to have evidence of extra-medullary disease 1 which is associated with a poor prognosis. In addition, clinical studies suggest that monocytic AML carries a greater risk for marrow and extra-medullary relapse after stem cell graft compared with non-monocytic subtypes 2, highlighting that there is an unmet medical need for effective treatment strategies for monocytic AML.
Antibody-drug conjugates (ADC) are emerging chemotherapeutic agents for treating cancers including AML 3. ADCs consist of antibodies (mAbs) conjugated with cytotoxic agents (payloads) through stable chemical linkers. Properly designed ADCs can selectively deliver payloads to target tumor cells, resulting in improved potency, broad therapeutic window, and prolonged circulation life compared with conventional drug classes for chemotherapy. Currently, “Mylotarg” is the first ADC approved for the treatment of newly diagnosed or refractory CD33-positive AML 4. Despite Mylotarg is a promising drug, it has been reported to have unexpexted safety issues; CD33 receptor is expressed not only in AML blasts, but also in normal hematopoietic stem cells; and its heterogeneous antibody-drug conjugation can lead to poor pharmacokinetics, efficacy and safety profiles. Then, it is necessary to find a specific target of AML cells to generate quality ADCs.
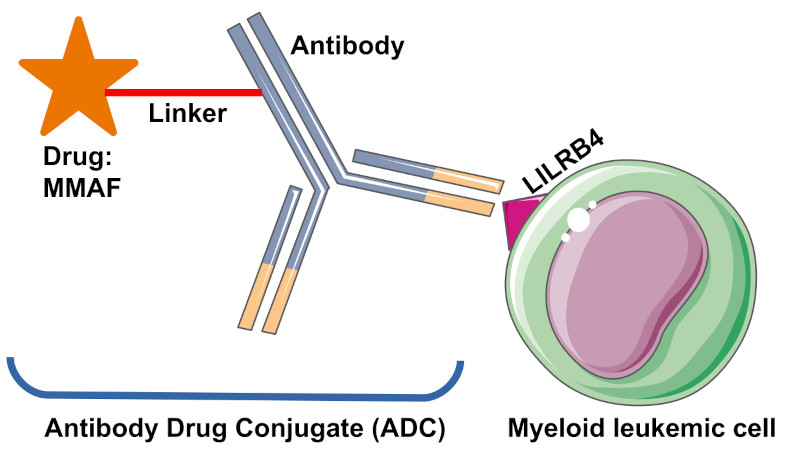
A promising target is the leukocyte immunoglobulin-like receptor subfamily B (LILRB) that is a group of type I transmembrane glycoproteins expressed by normal and malignant human cells of the myelomonocytic origin 5. Specifically, LILRB4 is expressed at significantly higher levels on monocytic AML cells than on normal counterparts, and its level inversely correlates with overall survival of patients with AML 678. Besides, LILRB4 supports tumor development by facilitating leukemia cell infiltration into tissues and by suppressing T-cell activity in AML cells. Thus, LILRB4 represents an attractive target for treating monocytic AML to achieve effective and safe targeted therapy. In the present paper, authors show the generation of anti-LILRB4 ADCs with high homogeneity, desirable physicochemical properties, high cell killing potency against AML cells, and marginal toxicity to normal progenitor cells. They also demonstrated that their conjugates exert a significant therapeutic effect in a mouse model of disseminated human AML. Thus, their findings highlight the clinical potential of their anti-LILRB4 ADCs in AML therapy.
Before proceeding to the development of the anti-LILRB4 ADCs, authors verified that the selected anti-LILRB4 mAb internalize upon binding to LILRB4 on leukemia cells. Once validated, they used the antibody-drug conjugation technologies developed by their group 910 to generate the anti-LILRB4 ADCs. The antibody drug conjugates are composed by three components: an antibody, a potent drug and the linker which connect the antibody with the drug through a chemical bond. Thus, firstly they introduced the linkers onto the anti-LILRB4 antibody and subsequently, they installed the antimitotic agent MMAF onto the linker conjugates. Finally, they corroborated that the constructed anti-LILRB4 ADCs bind with high affinity to human LILRB4.
Then, the authors checked the toxicity of the anti-LILRB4 ADCs using three AML cell lines with varying LILRB4 expression levels: THP-1 (AML, LILRB4++), MV4-11 (LILRB4+), and U937 (LILRB4-). As anticipated, MMAF alone did not show high potency or target specificity in either cell line and, importantly, ADCs could efficiently kill THP-1 and MV4-11 cells. As expected, the non-targeting ADC prepared from an isotype control (antibodies that lack specificity to the target) showed a marginal cell killing effect in these LILRB4-positive cells. Besides, U937 cells have no detectable LILRB4 expression, and correspondingly, no cytotoxicity was observed following incubation with the ADCs or the non-targeting ADC. Next, they evaluated the ADCs for potential on-target off-tumor toxicity using CD34+ umbilical cord blood cells. Remarkably, they found that ADCs did not affect precursor cells, highlighting that their anti-LILRB4 ADCs can selectively kill LILRB4-positive monocytic AML cells while sparing off-target progenitor cells.
Once they validated the specificity of the ADCs in vitro, they further evaluated the anti-LILRB4 ADCs in a mouse model of human AML. Seven days after immunosuppressed mice received AMLs cells intravenously; the conjugate and the control antibody weekly administered during the first three weeks. Interestingly, ADCs exerted therapeutic effect with statistically significant survival benefits and there were no obvious differences in body weight among the control and ADC treatment groups, suggesting that the ADC treatment did not cause significant acute toxicity. Besides, for pharmacokinetic evaluation of the ADCs in the body, they collected periodically blood samples and showed that the control antibody and the ADCs had comparable clearance rates, indicating that the rate at which they were removed from the body was similar.
In conclusion, the anti-LILRB4 ADCs demonstrated the capacity for LILRB4-mediated internalization, suitable physicochemical properties, and high cell killing potency against LILRB4-positive AML cells sparing normal progenitor cells. Besides, the anti-LILRB4 conjugates exerts a remarkable therapeutic effect and no significant toxicity in a mouse model of disseminated human AML. Thus, the findings highlight the clinical potential of anti-LILRB4 ADCs in monocytic AML therapy.
References
- Ganzel C, Manola J, Douer D, Rowe JM, Fernandez HF, Paietta EM, et al. (2016) Extramedullary Disease in Adult Acute Myeloid Leukemia Is Common but Lacks Independent Significance: Analysis ofPatients in ECOG-ACRIN Cancer Research Group Trials, 1980-2008. J Clin Oncol DOI: 10.1200/JCO.2016.67.5892. ↩
- Röllig C, Ehninger G (2015) How I treat hyperleukocytosis in acute myeloid leukemia. Blood DOI: 10.1182/blood-2014-10-551507. ↩
- Chau CH, Steeg PS, Figg WD. (2019) Antibody-drug conjugates for cancer. The Lancet DOI: 10.1016/S0140-6736(19)31774-X. ↩
- Godwin CD, Gale RP, Walter RB. (2017) Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia. DOI: 10.1038/leu.2017.187. ↩
- Kang X, Kim J, Deng M, John S, Chen H, Wu G, et al. (2016) Inhibitory leukocyte immunoglobulin-like receptors: Immune checkpoint proteins and tumor sustaining factors. Cell Cycle DOI: 10.1080/15384101.2015.1121324. ↩
- John S, Chen H, Deng M, Gui X, Wu G, Chen W, et al. (2018) A Novel Anti-LILRB4 CAR-T Cell for the Treatment of Monocytic AML. Mol Ther DOI: 10.1016/j.ymthe.2018.08.001. ↩
- Deng M, Gui X, Kim J, Xie L, Chen W, Li Z, et al. (2018) LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature DOI: 10.1038/s41586-018-0615-z. ↩
- Dobrowolska H, Gill KZ, Serban G, Ivan E, Li Q, Qiao P, et al. (2013) Expression of immune inhibitory receptor ILT3 in acute myeloid leukemia with monocytic differentiation. Cytometry B Clin Cytom DOI: 10.1002/cyto.b.21050. ↩
- Anami Y, Xiong W, Gui X, Deng M, Zhang CC, Zhang N, et al. (2017) Enzymatic conjugation using branched linkers for constructing homogeneous antibody-drug conjugates with high potency. Org Biomol Chem DOI: 10.1039/c7ob01027c. ↩
- Anami Y, Tsuchikama K. (2020) Transglutaminase-Mediated Conjugations. Methods Mol Biol DOI: 10.1007/978-1-4939-9929-3_5. ↩