Life Through the Looking Glass: An Unprecedented Threat?
Life Through the Looking Glass: An Unprecedented Threat?
Author: Ramón Muñoz-Chápuli has been Professor of Animal Biology in the University of Málaga until his retirement. He has investigated for forty years in the fields of developmental biology and animal evolution.
On December 12th, the British newspaper The Guardian published the following headline: “An Unprecedented Risk to Life on Earth“ The article wasn’t about asteroid impacts, climate change, or solar flares. Instead, it addressed something as exotic as “mirror life.” In other words, life as reflected in a mirror. Let’s explain this further.
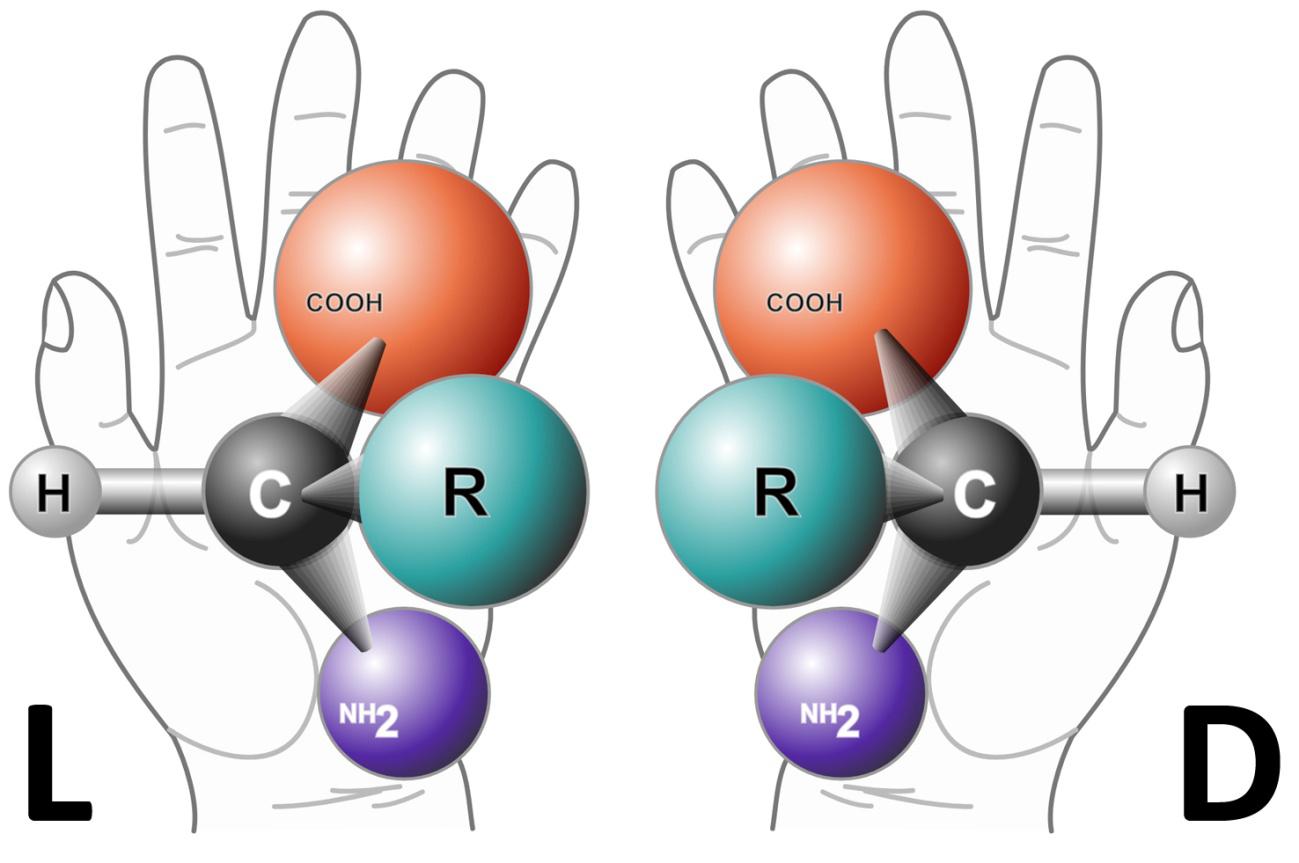
The molecules that make up proteins and nucleic acids can adopt two spatial configurations, known as L and D1. To understand this, we can look at our hands. They are different, cannot be superimposed, yet each is the mirror image of the other (Figure 1). This is why molecules with this non-superimposable property are called “chiral” (from the Greek cheirós, meaning hand).
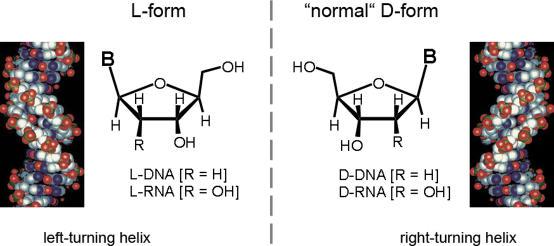
A surprising characteristic of life is that the amino acids that make up proteins2 are exclusively of the L form, with some exceptions found in bacteria. On the other hand, the nucleotides in DNA and RNA are of the D form (Figure 2). The reasons behind this bias remain enigmatic and have led to much speculation.
In nature, there are no proteins made from D-amino acids or nucleic acids composed of L-nucleotides. However, synthesizing these in the laboratory is perfectly feasible and is already being done, as described in a recent review 1. These compounds may have therapeutic applications. For instance, mirror proteins would be resistant to cellular degradation mechanisms and they would also avoid being processed by the cells responsible for presenting antigens to trigger an immune response. A notable example is the antagonist of vascular endothelial growth factor (VEGF-A) synthesized with D-amino acids. This molecule is stable, circulates in the blood for extended periods, and does not provoke an immune response, making it a potential candidate for anti-angiogenic therapies, such as inhibiting tumor vascularization.
The real possibility of synthesizing mirror proteins and nucleic acids is precisely what has raised alarms. In a recent issue of Science, a group of 38 researchers published a manifesto 2 calling for a ban on the production of cells, particularly bacteria, made up of mirror molecules. This group includes two Nobel laureates (Jack W. Szostak and Greg Winter) and the renowned Craig Venter, who in 2010 astonished the world by creating a bacterial strain with a completely synthetic genome 3.
The authors of the article, as well as an extensive technical report available online 4, argue the following: Sooner or later—perhaps within a decade—it will be possible to create artificial bacteria in the lab. If these bacteria are synthesized from mirror proteins and nucleic acids, they would evade the immune systems of all living beings: plants, fungi, and animals. They would also be resistant to bacteriophage viruses and bacterial predator microorganisms. Their spread in ecosystems and the threats to the health of living organisms would be unstoppable. Current vaccines, as well as chiral antibiotics (penicillins, tetracyclines, cephalosporins, vancomycin, etc.), would be ineffective. This is why mirror life is considered an unprecedented risk, a threat that should prompt immediate action to prevent such a scenario.
The authors of the article acknowledge the therapeutic potential of mirror proteins and do not see the need to restrict research in this field, as long as it does not lead to the creation of mirror life.
The researchers have not limited themselves to publishing their concerns in the form of an article and technical report. They have also created a platform (Mirror Biology Dialogues Fund), which will begin organizing events and activities starting next year.
The advances in contemporary biology are extraordinary and should continue to benefit humanity. However, it is also necessary to be aware of the risks posed by some of these advances and take action while there is still time.
Notes
1 From “levo” and “dextro”. The origins of these terms lie in the property of many chiral molecules to rotate the plane of polarized light to the left (L) or right (D). For organic molecules like amino acids or carbohydrates, this distinction is made based on the arrangement of groups attached to the chiral carbon.
2 Glycine, which is not chiral, is an exception.
References
- Harrison, K., Mackay, A.S., Kambanis, L. et al. (2023) Synthesis and applications of mirror-image proteins. Nat Rev Chem doi: 10.1038/s41570-023-00493-y ↩
- Katarzyna P. Adamala et al. (2024) Confronting risks of mirror life Science doi: 10.1126/science.ads9158 ↩
- Daniel G. Gibson et al. (2010) Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome Science doi:10.1126/science.1190719 ↩
- Adamala et al. Technical Report on Mirror Bacteria: Feasibility and Risks. December 2024. doi: 10.25740/cv716pj4036 ↩