A new theory on the early building blocks of life
From nucleic acids to proteins and sugar chains, all life is made up of polymers, large molecules made up of sequences of small units called monomers. However, how these macromolecules first appeared on the surface of Earth is still controversial. It is generally assumed that, right before life started, our planet was covered in very simple molecules such as water, carbon dioxide, methane, and ammonia. With the aid of energy and under the environmental conditions that existed at that time, simple reactions between them started generating small organic molecules, which gradually gave rise to more complex structures thanks to polymerization and replication (the process through which molecules make copies of themselves, thus generating primitive forms of genetic material). The question is how these processes happened without catalysts to drive them,1 given that enzymes (the catalysts of modern biochemical reactions) are very complex biopolymers themselves.
A possible answer to that question is that molecules we do not consider as life-related now may have been involved in the origin of life, and their different chemistries may have played a role in the first biochemical reactions. The latest to join this group of compounds are alpha hydroxy acids (αHAs), simple organic acids that contain a hydroxyl group bonded to the carbon atom adjacent to the carboxylic acid group (that is, the carbon atom in the alpha position, according to organic chemistry nomenclature).
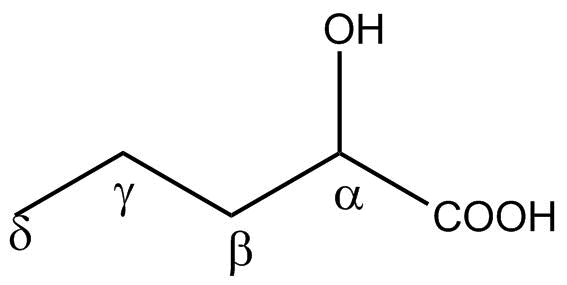
Recently, Kuhan Chandru and his coworkers2 have suggested that the polymerisation of αHAs may have had a role in the formation of living systems, similarly to the alpha-amino acids (αAAs) that make up modern proteins. Their hypothesis is consistent with the assumption that both αAAs and αHAs are likely to have been common in many primitive solar system environments, as they can be formed from the same mechanisms;3 in fact, lab simulations and the analysis of meteorites suggested that small αHAs and αAAs could be easily produced in those early life contexts. It has also been demonstrated that αHAs are able to polymerize more easily than amino acids under a variety of conditions, including those suggested to exist on primitive planets in the stages when life was originated.4 Besides, the fact that αHAs have been observed in many meteorites suggests that they may also have been efficiently delivered to the primitive Earth’s surface.5
Thermodynamic studies have shown that, under the same conditions, αHAs form longer oligomers in higher yield at much lower temperatures than αAAs. This is explained by the energy difference between the formation of an ester compared to that of a peptide bond (ΔG ~0 kcal/mol vs +3.5 kcal/mol), which makes the former much more feasible.6 The oligomerization of αHAs is energetically favourable, and a variety of polymers can form very rapidly, starting from a few different monomers. Once formed, the polymers are stable for significant amounts of time.
Since the environmental conditions on prebiotic Earth are still uncertain and several have been proposed,7 various microenvironments may have existed at the same time, and it has even been suggested that certain steps in the origin of life may have occurred off-Earth and then were brought by comets or other celestial bodies,8 the team made a mixture of five different αHAs react under a varied range of conditions of pH and temperature in order to simulate a variety of possible primitive environments, and then they analysed the results with a high-resolution mass spectrometer.
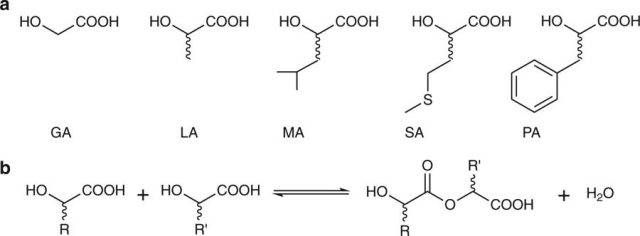
The reactions yielded hundreds of trillions of different polyesters. Just so that we have an idea of the numbers: for this experiment starting with five αHAs, there are 520 possible different combinations of monomers, only for 20-member linear chains. The fact that the stability of these polyesters also proved to be relatively higher than that of peptides under acidic conditions, led the authors to think that very early proto-organisms capable of producing or catching αHAs from the environment could have used them as the building blocks of polyesters, and that that αHAs may have preceded αAAs as the first building blocks of life, especially if some stages of chemical evolution occurred under relatively acid conditions. If a typical E. coli contains three different αHAs in concentrations of 1-2 mM, let’s imagine how many different polyesters could be generated in only one proto-cell with similar size and similar monomer concentrations, considering that, at equilibrium, this study detected oligomers made up of up to 22 units.
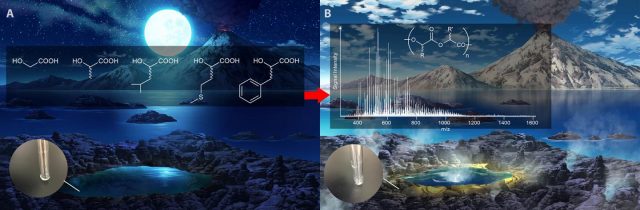
It is true that polyesters cannot form the regular secondary structures of polypeptides due their inability to form intra-molecular H-bonds,9 but they are still able to form folded 3-D structures that may have given them catalytic properties. Also, in further steps leading, side-chain functional groups may have been incorporated into αHAs, making these structures more and more complex and able to interact with other types of simple chemicals, possibly leading to self-replicating systems. This opens new insights in the chemistries that originated life.
References:
1 Cleaves, H. J, et al. “An evaluation of the critical parameters for abiotic peptide synthesis in submarine hydrothermal systems”. Orig. Life. Evol. Biosph. 2009, 39, 109.
2 Chandru, K. et al. “Simple prebiotic synthesis of high diversity dynamic combinatorial polyester libraries”. Communications Chemistry 2018, 1, 30. DOI: 10.1038/s42004-018-0031-1 10
3 Peltzer, E. T., et al. “The chemical conditions on the parent body of the Murchison meteorite: some conclusions based on amino, hydroxy and dicarboxylic acids”. Adv. Space Res. 1984, 4, 69.
4 Damer, B. & Deamer, D. “Coupled phases and combinatorial selection in fluctuating hydrothermal pools: a scenario to guide experimental approaches to the origin of cellular life”. Life 2015, 5, 872.
5 Pizzarello, S., et al. “A comparative study of the hydroxy acids from the Murchison, GRA 95229 and LAP 02342 meteorites”. Geochim. Comochim. Acta 2010, 74, 6206.
6 Meggy, A. B. “The free energy of formation of the amide bond in polyamides”. J. Appl. Chem. 1954, 4, 154.
7 Cleaves, H. J. “Prebiotic chemistry: geochemical context and reaction screening”. Life, 2013, 3, 331.
8 Pizzarello, S. & Shock, E. “The organic composition of carbonaceous meteorites: the evolutionary story ahead of biochemistry”. Cold Spring Harb. Perspect. Biol. 2010, 2, a002105.
9 Cleaves, H. J. “The origin of the biologically coded amino acids”. J. Theor. Biol. 2010, 263, 490.