Smelling the taste
When animals eat, both the olfactory (odour) and gustatory (taste) systems send information to the brain about the food being eaten. This combined information is very valuable to determine whether the animal is about to swallow something poisonous or something nutritious. That odours can influence the processing of taste is well known, and most people have experienced it when eating something while suffering a nasal congestion (e.g., during a common cold). The influence of the gustatory system on olfactory processing is less known, although evidence exists that taste can enhance odour detectability and perceived intensity. The brain areas where the gustatory system may exert this influence remain unidentified.
The classical view of the processing of the sensory information is that the primary sensory areas in the brain cortex receive unimodal information from the different senses, and then it travels to the association cortex where it is combined. Recent work has demonstrated, however, that primary sensory cortices receive multisensory influences and part of the information is thus combined “earlier” than previously thought. It is possible, then, that the influence of taste on the olfactory system extends to primary cortical regions.
In a recent article published in The Journal of Neuroscience 1, scientists from Brandeis University and the University of Utah (USA) recorded activity from single neurons in posterior piriform cortex (pPC)–primary olfactory cortex—of awake rats to determine whether taste influence on odour processing occurs at this level.
Authors showed that many of the pPC neurons responded to intraoral administration of basic taste solutions (sodium chloride, sucrose, citric acid, and quinine). These responses were selective, with some neurons responding to only one stimulus and some responding distinctly to the presentation of several taste stimuli. This selectivity indicates that it is unlikely that the responses are caused by the somatosensory system, which would have caused a non-selective response.
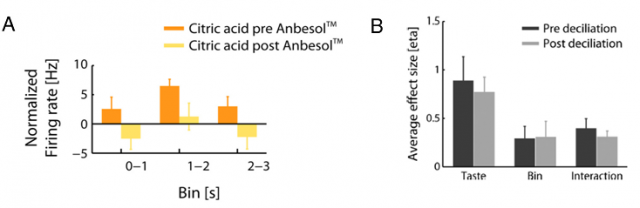
To confirm that these responses were truly from gustatory origin, and not caused by volatile components of the taste solution acting on the olfactory receptors, the authors designed different experiments. They demonstrated that applying Anbesol (an oral anaesthetic) directly to the tongue to inactivate gustatory receptors reduced pPC neurons response to the taste solutions (Figure 1A). However, when the authors removed olfactory cilia in the nasal epithelium to impair odour perception these responses were unaffected (Figure 1B).
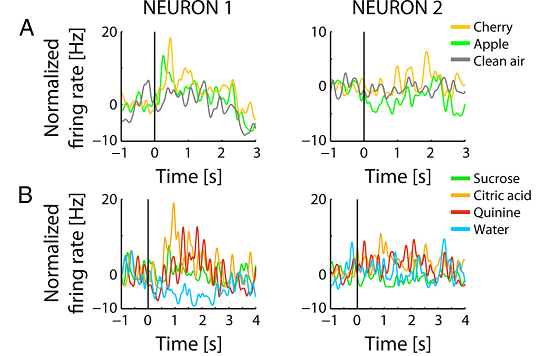
Finally, the authors showed that single neurons in the pPC receive both olfactory and gustatory inputs, so the convergence of the information about smell (Figure 2A) and taste (Figure 2B) occurs at the neuronal level.
In summary, this article provides a confirmation that taste may influence odour perception, and identifies an area in the primary olfactory cortex where this influence can happen. Additional research may be done to find the role of this area in the “flavour” network and its importance in the feeding behaviour.
References
- J.K. Maier, M. Wachowiak, D.B. Katz (2012) Chemosensory Convergence on Primary Olfactory Cortex. J Neurosci 32:17037–17047. DOI:10.1523/JNEUROSCI.3540-12.2012 ↩