A planar pentacoordinate carbon
The way a carbon atom binds to other atoms is what make this element so important. Apart from its capacity to bind to a wide range of different atoms, most remarkable is the way it can form long chains of carbon atoms with different kinds of covalent bonds between them . Biologically, for instance, the structure of biochemical molecules, their shape, depends primarily on the kind of bonds that the constituent carbon atoms form within the molecule; and this shape is so important that if a protein changes it, it may also lose its capacity to act as it was expected resulting in diseases and other metabolical problems.
All this is consistent with what we learnt at school: carbon can form 4 bonds, no more, no less. All of them could be single, then the structure would be tetrahedrical, as in methane, CH4; or one of them could be a double bond, then the structure would be a plane, as in ethylene, H2C=CH2; or it could be a triple bond, as in acetylene, HC≡CH, and we have a linear structure. In the case one of the 4 bonds is not formed, then we would have a very reactive and short-lived species called a radical, like H3C·
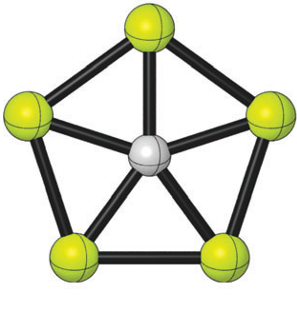
The impressive electronic properties of graphene also depend on the odd way carbon binds to three neighbouring atoms with single (σ) bonds, although in reality is a special double bond with all the atoms in the sheet bounded via a π bond, thus creating a conducting π–band. But, just think for a moment, what if we could combine de flexibility of carbon atom bonds with some elements with interesting characteristics? Could we not find some weird new molecules with some new exotic behaviours and potential applications in custom materials design? In 2008, Pei et al reported that a carbon atom could bind to 5 (yes, five) aluminium atoms producing a planar ion, CAl5+.
That same year Luo suggested that a different species, isoelectronic with CAl5+, i.e., with 18 valence-electrons, CBe54-, could also be a planar pentacoordinate carbon. But a tetraanion cannot be very stable due to the Coulomb repulsion and the eagerness to lose electrons. Now a group of researchers1, including Ivan Infante from DIPC, have found a way to get a stable planar pentacoordinate carbon from CBe54- that may be even possible to prepare experimentally in the gas phase. They publish their results in Physical Chemistry Chemical Physics.
The researchers have studied theoretically ways to make CBe54- stable using counter-ions that work very much in the same way buttresses do in architecture: providing support to act against the lateral (sideways) forces. In this case the sequential inclusion of lithium cations, maintaining 18 valence electrons around the carbon atom, on the CBe54- skeleton enhances de C-Be attraction. The election of lithium was based on the electronegativity difference between lithium and berylium and the propensity of lithium to be in a bridging position, thus a stable structure with a CBe5 pentagon could be expected, with lithium buttressing the Be-Be side.

The computational calculations confirmed that the stable (minimum global potential energy) forms of CBe5Li3-, CBe5Li22-, CBe5Li3–, CBe5Li4, CBe5Li5+ contain a planar pentacoordinate carbon. In all of this structures the carbon is apparently single bounded (σ bond) to five beryllium atoms, but not symmetrically, receiving charge from the Be atoms. But the carbon compensates this extra charge through a π-cloud, so that the clusters are doubly (σ and π) delocalized, as the magnetic field analysis suggests.
The fact that all this CBe5Linn-4 clusters with a planar pentacoordinate carbon are the lowest energy forms hints to the possibility that they could be obtained in the laboratory. Even though it would be technically very difficult, the experimental study of this compounds could show very interesting and unexpected properties.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Grande-Aztatzi R., Rafael Islas, Ivan Infante, José M. Mercero, Albeiro Restrepo & Gabriel Merino (2015). Planar pentacoordinate carbons in CBe 5 4− derivatives , Phys. Chem. Chem. Phys., 17 (6) 4620-4624. DOI: http://dx.doi.org/10.1039/c4cp05659k ↩