Carbon nanotubes as shields to enhance photocatalysis
Author: Daniel González-Muñoz is a predoctoral researcher in photocatalytic processes at Universidad Autónoma de Madrid.
We live in a time when scientific applications are growing by leaps and bounds. This exponential growth has been possible, among other tools, thanks to the application of nanotechnology. There is something that nanotechnology has taught us: In science, size matters. When studying matter at nanometric levels, we find that the properties are completely different from the macroscopic world, being able to improve the scientific applications of today.

Among all the nanoparticles that have been developed in recent decades, carbon nanotubes (CNTs) are of special interest. CNTs are the result of folding a sheet of graphene to form a cylinder, and they are interesting due to their physicochemical properties, such as their conductivity. There are different types of CNTs, and this study is focused on single walled carbon nanotubes (SWNTs), which are carbon nanotubes made up of a single cylinder. The main objective of this work is to functionalize SWNTs with an organic photocatalyst, so that a heterogeneous photocatalyst capable of being recycled is designed 1.
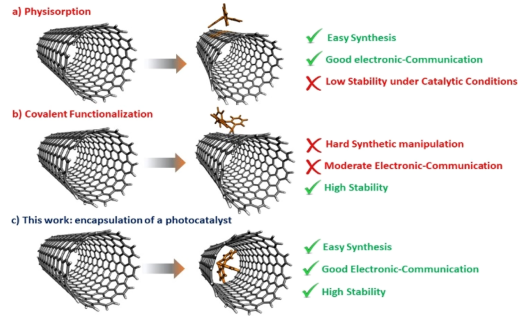
The chosen organic photocatalyst is 10-phenylphenothiazine (PTH), an organic photocatalyst widely used in organic synthesis, since it is able to reduce challenging substrates (E°red = -2.1 V vs SCE). On the other hand, PTH has the disadvantage of absorbing exclusively in the UV region of the solar spectrum, and being an organic molecule, it is degraded easily. Thus, there are three possible ways to functionalize SWNTs with PTH (Figure 1). The first is physisorption (Figure 1 (a)), which consists of joining PTH with the outer walls of SWNTs through non-covalent bonds (weak bonds). This strategy is carried out with simple synthesis methods and allows a good electronic communication between photocatalyst and nanotube (due to the proximity between them), but they tend to be not very stable under catalytic conditions, since the bonds that form the hybrid photocatalyst are weak. The second strategy is covalent functionalization (Figure 1 (b)). Unlike physisoprtion, this strategy allows high stability under reaction conditions, but it often requires laborious synthesis methods and the communication between photocatalyst and nanotube may decrease. Finally, there is a third strategy known as endohedral functionalization (Figure 1 (c)). This strategy consists of introducing PTH into the interior cavity of the SWNT. This strategy combines the advantages of physisoprtion and covalent functionalization, keeping the structure of the photocatalyst unchanged.
It may seem difficult to know if the photocatalyst (PTH) is inside the carbon nanotube, or on the outside surface. For this reason, one of the most enlightening techniques is Raman spectroscopy (Figure 2, top, left). To demonstrate that PTH is inside the SWNT cavity, three representative samples are prepared. oSWNT (3) are carbon nanotubes before functionalizing them with PTH photocatalyst. PTH-cSWNT (4) are carbon nanotubes with closed tips. In this way, when functionalizing carbon nanotubes with PTH, the functionalization is on the outer surface. The last sample, PTH@oSWNT (5), corresponds to the endohedral functionalization of SWNTs with PTH photocatalyst. In the spectrum the radial breathing mode (RBM) region is highlighted. The RBM region represents how the carbon nanotube structure expands and contracts. When a molecule is inside the cavity, new signals appear around 250 cm-1. This occurs in the PTH@oSWNT sample (5), which demonstrate that PTH molecule is inside the carbon nanotube. In figure 2 (Top, right), it is showed thermogravimetric analysis (TGA). TGA analysis shows the degree of functionalization inside SWNTs. In PTH@oSWNT, a mass loss between 300-500 ºC is observed, which corresponds to the degradation of the organic photocatalyst PTH. This degradation corresponds to 8% functionalization inside carbon nanotube.
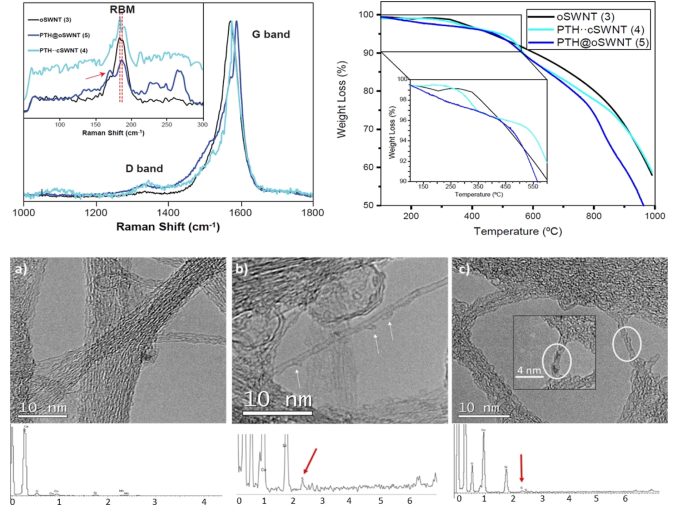
Another characterization technique capable of discerning if the photocatalyst is inside the carbon nanotube is transmission electron microscopy (TEM) (Figure 2, bottom). In PTH-cSWNT (4), the photocatalyst adhered to the outer walls of the nanotube (Figure 2b) is observed, while in PTH@oSWNT (5) the photocatalyst can be seen inside the cavity (Figure 2c). To finalize the characterization, absorption and emission studies of the photocatalyst PTH @ SWNT (5) were carried out. As mentioned above, carbon nanotubes are conductive nanostructures, and the presence of the PTH photocatalyst inside the cavity causes it to undergo a bathochromic shift in its absorption towards visible light. In this way, the use of UV light to excite the photocatalyst is avoided.
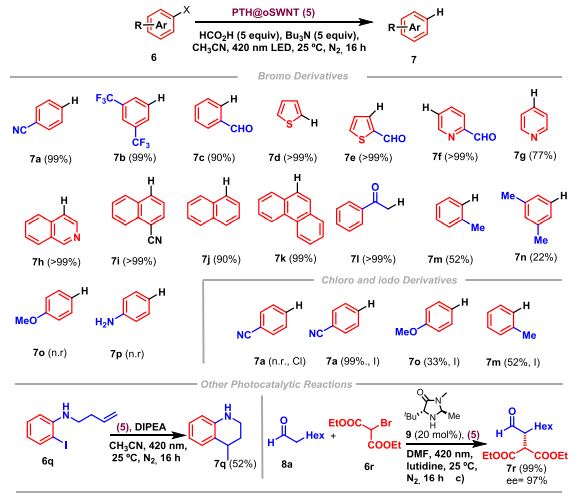
Once the PTH@oSWNT (5) photocatalyst is characterized, its efficiency as photocatalyst in different organic reactions can be demonstrated. PTH@SWNT (5) is able to dehalogenate aromatic halides with different substituents in the aromatic ring, both electron donating and electron withdrawing groups (Figure 3, 7a-p). Furthermore, the photocatalyst is capable of carrying out the synthesis of tetrahydroquinolines (Figure 3, 7q) and the α-alkylation of aldehydes (Figure 3, 7r), two important reactions in the synthesis of drugs. It is important to add that, by synthesizing the heterogeneous photocatalyst PTH@SWNT (5), it is capable of being recycled. This allows the same photocatalyst to be used for eight consecutive reactions, without losing catalytic activity.
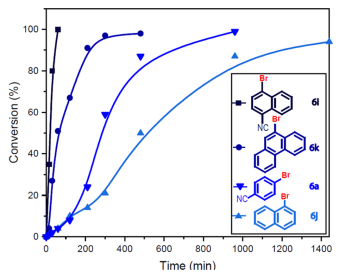
The conductive ability of carbon nanotubes can be further exploited in organic synthesis reactions, and this is the really interesting part of this work. When the PTH photocatalyst is excited in the inner cavity of the carbon nanotube, it is capable of injecting an electron into the system of the nanotube wall. This makes charge separation very effective, leading to greater catalytic activity. To demonstrate this fact experimentally, the debromination of several substrates with different numbers of aromatic rings is carried out. In this way, the substrates that present more aromatic rings have a greater interaction with the wall of the nanotube, leading to an increase in the reaction rate. As can be seen in figure 4, substrate 6k is able to react faster than its analog (6j) with one less aromatic ring. Substrate 6a has a cyano group in the -para position, which decreases the reduction potential of the molecule, and leads to a higher reaction rate. This explains the higher reaction rate of substrate 6a compared to substrate 6j, even though 6j has one more aromatic ring. However, Substrate 6l, which has one more aromatic ring than its analog 6a, has a much higher reaction rate, demonstrating the influence of interaction with the walls of the nanotube.
Carbon nanotubes are capable of incorporating photocatalysts into their structure to form heterogeneous photocatalysts. The great advantage of carbon nanotubes is that they are conductive nanostructures, which can greatly improve electron flow and catalytic activity. Nanotechnology has countless applications in photocatalysis, and in the coming years we will see great advances in the development of new photocatalysts.
References
- Daniel González-Muñoz, Ana Martín-Somer, Klara Strobl, Silvia Cabrera, Pedro J. De Pablo, Sergio Díaz-Tendero, Matías Blanco and José Alemán (2021) Enhancing Visible-Light Photocatalysis via Endohedral Functionalization of Single-Walled Carbon Nanotubes with Organic Dyes. ACS Appl. Mater. Interfaces. 13, 24877-24886. doi: 10.1021/acsami.1c04679 ↩