A new path to enantioselective substituted pyrrolidines
Imagine a very simple chemical estructure: 4 carbon and 1 nitrogen forming a ring, with no double bonds, just simple bonds between any two of them and with hydrogen. This compound is called pyrrolidine and is the base of very important biological and farmacological products.
Pyrrolidine is found naturally in the leaves of tobacco and carrot. The pyrrolidine ring structure is present in numerous natural alkaloids such as nicotine and hygrine. It is found in many pharmaceutical drugs such as procyclidine and bepridil. It also forms the basis for the racetam compounds (e.g. piracetam, aniracetam). A pyrrolidine ring is the central structure of the amino acids proline and hydroxyproline. 1
Thus, the pharmacological (and, therefore, economical) importance of pyrroline based compounds is paramount, being one of the critical points obtaining the right optical isomer (or enantiomer). As the substituted pyrrolidine ring will likely have no plane of symmetry, different configurations with different optical activity will appear, each one called an enantiomer. And since many biological molecules are enantiomers, there is sometimes a marked difference in the effects of the two enantiomers of a potential pharmacological candidate on biological organisms.
As a consequence different routes to get the right enantiomer of pyrrolidine-based compounds have been considered. Owing to its intrinsic atom-economical nature, the catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides is one of the most straightforward approaches for the enantioselective synthesis of substituted pyrrolidines. These reactions are highly stereo– and regioselective, and have the potential to form four new contiguous stereocenters. Consequently a great effort has been put in optimizing the efficiency and scope of this reaction, mainly searching for appropriate catalysts.
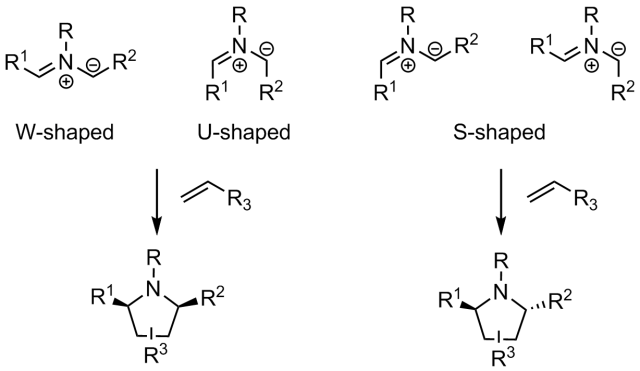
As a result, a wide variety of chiral metal catalysts and organocatalysts have been developed. In spite of this progress, the range of suitable dipolarophile partners is still limited, being typically restricted to alkenes activated with strongly electron withdrawing substituents, such as carbonyl, nitro, sulfonyl, cyano, and phosphonate groups.
The use of alkylarenes (any aliphatic-substituted aromatic hydrocarbon) in the catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides has hardly been studied at all. This lack of precedent is even more acute in the case of compounds where the aliphatic radical is vynil, like phenylethene and all the family of styrenes, the use of which in catalytic asymmetric 1,3-dipolar cycloaddition with azomethine ylides remains to be described.
Now, Ikerbasque professor Abel de Cózar and Fernando Cossío, from UPV/EHU and DIPC, in collaboration with researchers from Universidad Autónoma de Madrid, have investigated 2 whether vinylarenes would be suitable partners in this process, which would enable practical enantioselective access to 4-arylsubstituted pyrrolidines.
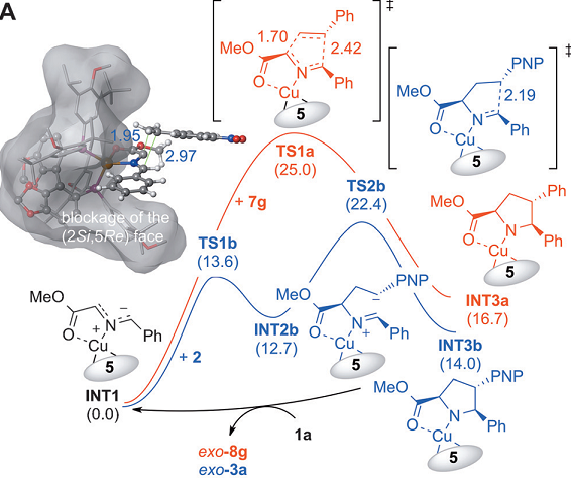
The researchers show that the behaviour of vinylarenes in the catalytic enantioselective 1,3-dipolar cycloaddition of azomethine ylides depends on the catalytic system used. Under appropriate reaction conditions with a CuI or AgI catalyst either the exo or the endo adduct was obtained with high stereoselectivity.
Computational studies showed how electronic effects in the dipolarophile can result in a change of mechanism and in very efficient polarization of the electrophilicity towards the terminal carbon atom. This process provides compelling evidence that modestly activated olefins are suitable dipolarophiles in the catalytic asymmetric cycloaddition of azomethine ylides and is an efficient access to highly enantiomerically enriched 4-aryl proline derivatives.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
References
- Pyrrolidine PubChem-National Center for Biotechnology Information ↩
- Ana Pascual-Escudero, Abel de Cólzar, Fernando P. Cossío, Javier Adrio, and Juan C. Carretero (2016) Alkenyl Arenes as Dipolarophiles in Catalytic Asymmetric 1,3-Dipolar Cycloaddition Reactions of Azomethine Ylides Angewandte Chemie International Edition doi: 10.1002/anie.201609187 ↩