Tumor Killer Viruses

Killing cancer cells is a key point to cure cancer. Many researchers are working on developing different therapies to do just that. The one I’m going to talk about today is a very promising one: Oncolytic Virotherapy. Just the name may scare you but it’s a very clever approach to treat some tumors lacking a good treatment.
This therapy uses viruses (oncolytic viruses) that infect and damage tumoral tissues without harming normal tissues 1. These viruses use different mechanisms to kill the infected cancer cells. For instance, they can cause the direct cell break or produce the cell death through the activation of some immune system cells. In addition, they can promote the death of uninfected cancer cells by indirect mechanism such as amplification of specific anticancer immune responses or destruction of blood vessels.
But let’s start from the beginning. In the 1950s (when tissue culture systems and mice cancer models started to be developed) some researchers started to use viruses to kill cancer cells. Many cancer patients were treated with impure oncolytic virus preparations administered in many different ways. Most of the times the immune system of the patients stopped the viruses and didn’t affect tumor growth but in some immunosuppressed patients their tumors decreased. However these patients got sick and died when the infection spread to normal tissues. Nowadays, oncolytic viruses are engineered to suppress their pathogenicity and toxicity and to target just cancer cells.
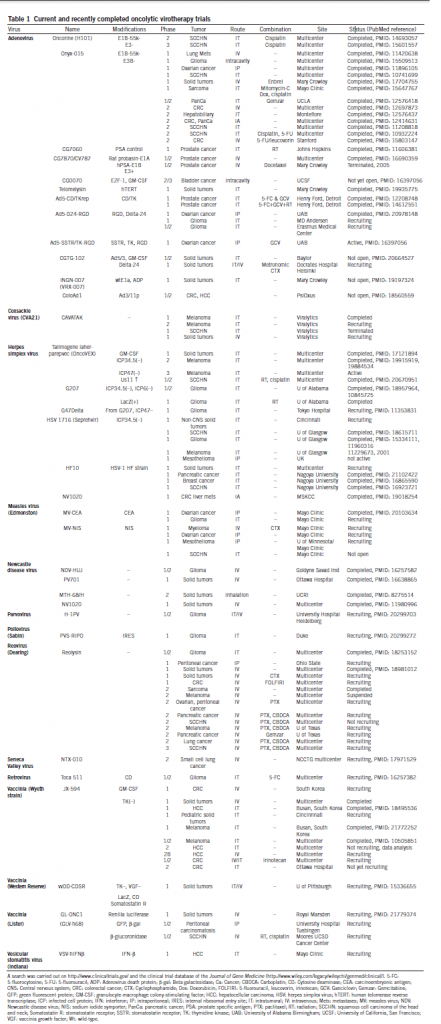
In 1991 some researchers published a study in which a Herpes Virus Simplex with attenuated neurovirulence was shown to be active in a mouse model of glioblastoma. Since that moment the clinical activities in the oncolytic virotherapy field have increased a lot and nowadays there are many ongoing and finished clinical trials with oncolytic viruses (Table 1). Most of the trials are done using intratumoral injection as delivery method of the virus into the tumor. However, systemic delivery will be required for treatment of metastatic cancer. The problem of systemic delivery is to get the viruses into the cancer cells. For this, researchers are trying to find new ways of minimizing oncolytic virus sequestration in the liver and spleen, evading neutralisation by serum factors, targeting viruses to the vascular endothelial cells lining tumor blood vessels and selectively enhancing vessel permeability (Figure 1). As you can imagine oncolytic virotherapy is a fast changing field and when a virus makes it to a clinical trial new state-of-the-art ones appear.
But why do I bring this subject here today? Because in the last months of 2012 one if these viruses has been granted the status of Advanced Therapy Medicinal Product by the European Committee of Advanced Therapies. This virus is a Herpes Simplex Virus 1 (HSV-1) without the ICP34.5 and ICP47 genes and the insertion of the granulocyte macrophage colony-stimulating factor (GM-CSF) gene 2. HVS-1 is an enveloped dsDNA virus that causes common fever blisters in humans; it can enter eukaryotic cells and induce a lytic effect, and has some advantages over other oncolytic viruses. The ICP34.5 gene is a neurovirulence factor and eliminating it from the virus removes its neurotoxicity. The ICP47 gene blocks antigen presentation and its elimination helps to generate a tumor-specific immune response. In addition, the presence of the GM-CSF gene promotes the maturation of antigen-presenting cells such as dendritic cells and stimulates cellular immune responses.
Taking all this into account, this virus, called talimogene laherparepvec (formerly OncoVEX), has two main effects. It induces lytic tumor cell death by selectively viral replication in tumor cells and produces the induction of an anti-tumor immune response, a process that is enhanced by the local expression of GM-CSF. The approved indication for this virus is metastatic melanoma and phase II clinical trials have been completed in patients with unresectable (without possibility of removing the lesion by surgery) metastatic stage III and IV melanomas. In phase I and II trials this virus was well tolerated giving only minor side effects in the injection point or flu-like symptoms. These side effects were really small taking into account the benefits for the patients. The main results of the phase II trial (with 50 patients)3, were that, overall, 20% of patients achieved complete response without additional surgery and 26% had no evidence of disease with three patients getting surgery. This was the primary endpoint of the trial. For the secondary endpoint, the study showed that the median overall survival was greater than sixteen months for all patients and the overall 2-year survival was 52%. In patients with stage III and IV melanomas this survival rates are really good because the 1-year overall survival of patients with these melanomas treated with other drugs is around 10 to 15%. The results of these trials are very promising and a phase III trial, which will be completed in the first months of 2013, has already started 4.
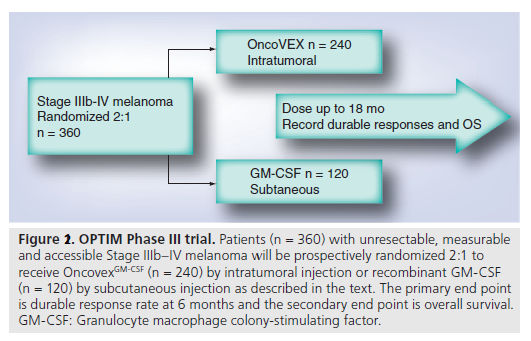
The phase III clinical trial with this virus is an atypical one (Figure 2). One of the reasons is that this trial uses GM-CSF as a control, instead of Dacarbazine, the typical control in melanoma trials. On the other hand, using GM-CSF will help find out its role in the treatment. Another problem that some experts see in this trial is that this virus will be useful only for few patients, those ones who have an injectable lesion, such as melanoma on the surface of the skin, in the soft tissue below the skin and the lymph nodes. These are only a third of advanced melanoma patients. Nevertheless, the study’s primary endpoint is a statistically significant improvement in durable response rate (defined as the rate of complete response or partial response lasting continuously for six or more months) and the secondary endpoint (overall survival) will be reported when the trial is finished.
Even with all the doubts experts have, mainly about the immunogenic activity of this virus (due to the fact that the injection is intratumoral and it will be difficult for the virus to produce a distant effect in metastatic lesions), the results of the talimogene laherparepvec (OncoVEX) clinical trials are very exciting, and after the approval of this virus as an advanced therapy product, its production and commercialisation may be closer than we think, increasing the therapeutic tools to treat this kind of cancer and others in the future.
References
- Russell et al. Oncolytic Virotherapy. Nature Biotechnology (2012) 30(7):1-13 ↩
- Sivendran et al. Herpes simplex virus oncolytic vaccine therapy in melanoma. Expert Opin. Biol. Ther.(2010) 10(7):1145-1153 DOI: 10.1517/14712598.2010.495383 ↩
- Kaufman et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. (2010) 17:718-730 DOI: 10.1245/s10434-009-0809-6 ↩
- Kaufman and Bines. OPTIM trial: a phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. (2010) 6(6):941-949 DOI: 10.2217/FON.10.66 ↩