Breaking the wall so immunotherapy reaches the heart of the tumor
Author: José R. Pineda got his Ph.D. from University of Barcelona in 2006. Since 2007 he has worked for Institut Curie and The French Alternative Energies and Atomic Energy Commission. Currently he is a researcher of the UPV/EHU. He investigates the role of stem cells in physiologic and pathologic conditions.
Solid cancerous tumor is a localized tumor mass that can invade neighboring tissues or even migrate to other parts of the body, forming metastasis. The evolution of cancer is determined by the tumor genetic mutations and the interaction with the tumor microenvironment. Tumor microenvironment is composed of healthy cells such as immune cells, blood vessels that will nourish the tumor, and supporting cells called “fibroblast stromal cells” (a type of cell derived from mesenchyme) that the cancer has hijacked to its advantage. In healthy conditions, fibroblast stromal cells form a framework of connective tissue in all organs secreting extracellular matrix, such as collagen, and regulatory factors. These cells ensure the proper functioning of organs and play an essential role in tissue repair.
Lucie Peduto’s team, from the Pasteur Institute, published a work led by Di Carlo and collaborators describing the role of a subpopulation of stromal cells that forms a protective matrix cocoon around the tumor with hypoxic and immunosuppressive characteristics. In the same way that there is the doubt about “what came first, the chicken or the egg”, targeting stromal cells was considered risky because this cocoon protects its host from the tumor. However, their recent findings highlights that this barrier also protects the tumor during the first stages of tumor growth from the immune system… 1. Indeed, recent strategy for tumor treatment known as “immunotherapy” unfortunately still prove ineffective against solid tumors. These fibroblast stromal cells seem to block the access of cytotoxic lymphocytes to the tumor site, preventing them from killing malignant cells.
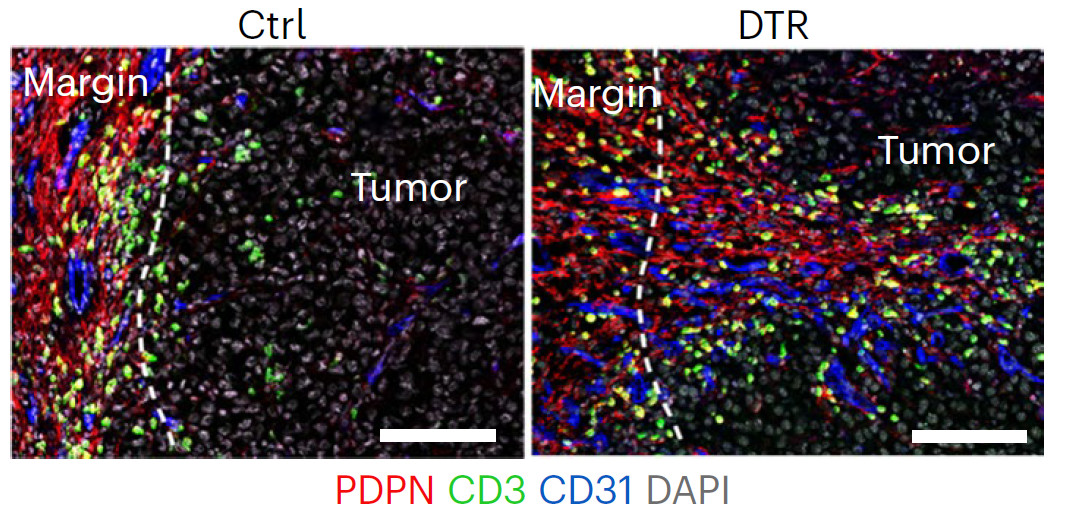
Di Carlo and collaborators identified a new mesenchymal stromal cell population (MSCs) which develops around the tumor and allows it to escape the immune system. Unlike the “classic” stroma, they found that this stromal population express a surface protein called ADAM12 and regulate the macrophages and the vascularization of the tumor, controlling the influx and activation of cytotoxic lymphocytes at the tumor site. To demonstrate that, they injected MSCs from genetically engineered mice expressing ADAM12-GFP (ADAM12 tagged with green fluorescent protein) together with B16-OVA melanoma cells (they called them as “MO5”). They found that ADAM12-GFP was localized at the tumor margin. Interestingly, the frequencies and absolute numbers of ADAM12+ cells decreased at later tumor stages. To deplete ADAM12+ MSCs, they inoculated MO5 melanoma cells in ADAM12-DTR mice (mice that diphtheria toxin receptor (DTR) is expressed under the control of the Adam12 promotor), provoking the death of significant numbers of ADAM12+ cells after toxin administration (Figure 1). This strategy led to unveil the role of this particular cell subpopulation during MO5 melanoma progression, finding a reduction of 50% of the tumor growth and more infiltration of CD8+ T cells and natural killer (NK) cells but no effect during the in initial stages of the development of tumors. To confirm that, treatment with CD8+ T cell-depleting antibodies restored tumor growth in the absence of ADAM12+ cells, confirming that ADAM12+ cells block antitumor activity of CD8+ T cells.
During tumor evolution, carcinoma associated fibroblasts (CAFs) migrate intratumorally and settle near blood vessels. RNAseq gene expression analysis from poorly infiltrated tumors or from tumors lacking ADAM12+ cells (which were highly infiltrated) identified Car9, Saa3, Lcn2, Mmp9 and Mmp13 genes, strongly induced by hypoxia that were significantly downregulated in the CAFs of tumors lacking ADAM12+ cells. Tumor hypoxia results mainly from poorly functional collapsed tumor vasculature that lacks pericyte coverage. They found that in tumors lacking ADAM12+ vasculature had both pericyte coverage and presence of ICAM1 (a surface molecule essential for leukocyte adhesion and infiltration) suggesting that depletion of ADAM12+ MSCs was able to normalize CAFs by decreasing tumor hypoxia (restoring oxygen levels). Then, to investigate the underlying mechanism, they performed a transcriptome analysis of ADAM12+ cells isolated from MO5 melanomas, observing the biggest differences in extracellular matrix (ECM) remodeling, cell proliferation, cell differentiation, and inflammation. ADAM12+ cells expressed high levels of Tgfbr2 (a receptor of the superfamily of TGF-beta signaling pathway). Using a Cre/Lox genetically engineered mouse model for Tgfbr2 ablation in ADAM12+ cells they found, at early tumor stages, an increased infiltration of T cells and a reduction of tumor growth respect to control animals. These results concluded that TGFBR2 is required for the pro-tumorigenic role of ADAM12+ cells.
Other interesting discovery characterizing tumors lacking ADAM12+ cells was that the genetic profile of infiltrated macrophages expressed lower levels of Tgfb1 (ligand of Tgfbr2 receptor and potent immunosuppressor) and upregulated the molecule “Light (Tnfsf14)” that promote activation of NK and T lymphocytes (antitumoral immunity) and restore blood vessels integrity, concluding that ADAM12+ cells promoted immunosuppression.
Because ADAM12 is a poor prognosis marker and has been found to be overexpressed in other lethal tumors rather than melanoma, such as prostate, pancreas, breast and colorectal, among others, the authors decided to compare whether the results observed in melanoma could be extrapolated to other types of human tumors. Using public databases and crossing the information, they found that ADAM12 expression stratifies patients harboring tumors with high levels of hypoxia, inflammation and innate resistance mechanisms, recapitulating what they observed in their preclinical study for MO5 tumors. Interestingly, tumor-induced ADAM12-expressing stromal cells are also present when one is injured. However, the tumor hijacks their repair function to protect itself. By promoting the action of tumor macrophages, these cells will inhibit the immune system and lead to the creation of new dysfunctional blood vessels, which develop in an anarchic manner. This result limits the influx of antitumor immune cells (or therapeutic antibodies during immunotherapy), creating a hypoxic environment and inhibiting the activity of antitumor immune T and/or NK lymphocytes.
Future strategies to target and destroy ADAM12+ stromal cells could be a concomitant therapeutic option to facilitate access to the tumor site for activated cytotoxic lymphocytes or improve CAR-T therapies for solid tumors such as pancreatic, breast or prostate cancers.
References
- Selene E. Di Carlo, Jerome Raffenne, Hugo Varet, Anais Ode, David Cabrerizo Granados, Merle Stein, Rachel Legendre, Jan Tuckermann, Corinne Bousquet & Lucie Peduto (2023) Depletion of slow-cycling PDGFRα+ADAM12+ mesenchymal cells promotes antitumor immunity by restricting macrophage efferocytosis Nature Immunology doi: 10.1038/s41590-023-01642-7 ↩