Against a failing heart, pharmacoepigenomics
The last advances in genetics and genomics are leading a change in the paradigm about how to treat diseases. Epigenetics is the science field that studies how gene expression is regulated. Several modifications on the genes such as DNA-methylation, histone-acetylation or microRNA (miRNA) regulation are considered to be the main responsible in controlling gene expression changes (figure 1), and thus, a new focus of attention for new therapeutic strategies. Pharmacoepigenomics, which could be defined as the science field that studies the use of drugs (or the search of new therapeutic targets) to modify gene expression, appears on the scene.
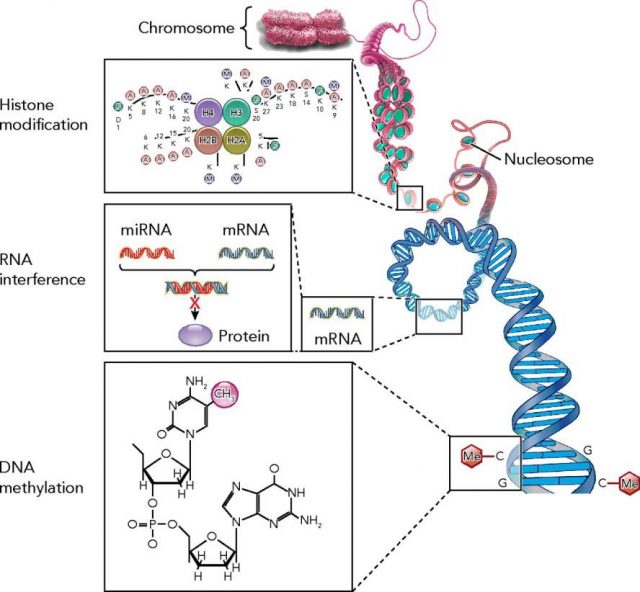
Drugs or gene therapies that would modify (inhibiting or activating) epigenetic mechanisms could come to the real word very soon to treat those diseases where gene expression changes play an important role.
In a recent paper published in Nature 1, an inhibitor of the microRNA miR-25 has been postulated as a potential new therapeutic strategy to rescue failing hearts by improving cardiac contractility. miRNAs are small ribonucleic acids chains that, when bound to specific complementary DNA sequences within the promoter region of a particular gene, will inhibit its expression. In health and disease, these miRNAs control the expression of genes involved in every physiological or pathological function. Some of these miRNAs are overexpressed in heart failure, and as a consequence, the expression of several genes involved in cardiac contractility is repressed.
The use of miRNAs as a therapy to inhibit the expression of an overexpressed gene is one of the approaches that scientist in the field are exploring nowadays. However, in this paper the situation is the opposite. The purpose was to stimulate the expression of a particular gene (the sarcoplasmic reticulum calcium uptake pump SERCA2a), which is mainly controlled by miR-25. How to do that? The answer seems clear for the authors: by inhibiting the activity of this particular miRNA. SERCA2a controls the calcium uptake by cardiomyocytes, and this calcium is an essential element necessary for heart contraction, and so for normal cardiac activity. The expression of SERCA2a is decreased and its activity is reduced in the failing heart thus compromising cardiac function.
In the paper, Wahlquist and co-workers had screened more than 800 miRNAs that could potentially interact with SERCA2a. Four of them caused a highly significant prolongation of the decay (re-uptake) phase of the calcium transient in a cardiomyocyte cell line, an assay used to evaluate the activity of SERCA2a. What they really did, was to culture these cardiomyocytes in a petri dish and to stimulate them with a single 15V electrical pulse of 5ms at 1 Hz, recording the response with a microscope and the help of a fluorescent marker for intracellular calcium2. Fluo-4 is a fluorescent marker which gives you a green light when binding to calcium thus giving you a quantifiably idea on how quick and how much this cation is being released inside the cell. SERCA2a is the primary mechanism for calcium re-uptake into the stores during excitation-contraction coupling. An electrical pulse will act as an initial excitatory effect, opening gates for calcium to get inside the cell. This calcium will activate SERCA2a that will re-uptake it into the sarcoplasmic reticulum (the calcium stores for cells). An inhibition of the expression-activity of this transporter would be evidenced by an extension in the time to re-uptake calcium, measured by the time green fluorescence disappears.
Among those four, the most potent miRNA was miR-25, which elicited a physiological effect comparable to that of the short interfering RNA (siRNA, a common technique to block gene expression in cell culture) directed against SERCA2a. In other words, miR-25 inhibited the expression of the calcium pump to the same level than when external reagents are used to knockdown this particular gene in vitro. This miR-25 was also found up-regulated in failing hearts, and more specifically in cardiomyocytes conferring it selectivity among cardiac muscle. miRNAs can block the expression of several genes in one or more different tissues. To explore the specificity of miR-25 against the calcium pump, the authors induced a targeted down-regulation using a SERCA2a siRNA. The use of a siRNA against SERCA2a mimicked the effect of miR-25. In mice, a selective overexpression of cardiac miR-25 decreased expression of the calcium pump and deteriorated in vivo cardiac activity, while others did not cause any effect. All these facts confirmed that the main effect of this particular miRNA is due to specific inhibition of the calcium pump gene expression.
Once confirmed the role of miR-25 in deteriorating contractility of the failing heart, it was necessary to evaluate the physiological effect of blocking it in an animal model (mice) by using an AntagomiR as a therapeutic agent. These molecules are short complementary or antisense RNA sequences that bind to specific miRNAs, thus blocking its capacity to bind and regulate gene expression. Overall, they have an opposite effect, they have an antagonistic effect. These miRNAs can have deleterious effects on one tissue (as occurs in the failing hearts) but they can be necessary in the rest of the body. Specific delivery of the Anti-miR-25 is essential to avoid undesirable secondary effects if we would like to test it as a potential therapy in human. This is why the oligonucleotide was encapsulated inside a virus, a tissue-specific virus. During infection, the virus will release its content, the anti-miR-25 in this case, inside the cell. The use of modified viruses is a common practice in research to specifically target the delivery to a particular tissue. In this case, the authors used an adenovirus, the adeno-associated virus 9, which selectively target the heart. The adeno-associated virus infects human cells but do not cause any disease, being a perfect vector for its use in gene therapy.
The intravenous injection of the adeno-associated virus 9 containing anti-miR-25 induced a reduction of miR-25 levels with a subsequent increase in the myocardial expression of the calcium pump SERCA2a in mice hearts. These consequences resulted in a substantial improvement of cardiac function during heart failure when compared with non–treated animals. Following this work, authors conclude that “inhibition of miR-25 may be a novel therapeutic strategy for the treatment of heart failure”.
Indeed, the use of adeno-associated virus has been approved for human use. Several clinical trials are currently running in phase I (Screening for safety), phase II (Establishing the efficacy against a placebo), and even one has reached phase III (Final confirmation of safety and efficacy) to treat lipoprotein lipase deficiency and has received marketing approval from the European Medicine Agency3. Among these trials, one was designed to treat severe Heart Failure, against the same target that the exposed work. In this trial, called CUPID (acronym from Calcium Up-regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease) Jessup and co-workers used adeno-associated virus to directly overexpress SERCA2a in failing hearts from patients in late phases of the disease. This phase II clinical trial demonstrated safety and suggested benefit in advanced heart failure, supporting larger confirmatory trials4.
Even though the effective and tissue specific delivery of this genetic material will continue to be a major challenge in the research field, the future of this kind of ‘new therapies’ looks very promising. Both miRs, anti-miRs via gene therapy, and drugs to modulate histone-acetylation or DNA-methylation are getting more in use nowadays. Drugs such as Vorinostat (suberanilohydroxamic acid, a histone deacetylase inhibitor) has been already approved by the American Food and Drug Administration for Cancer treatments and suggested for other cardiovascular diseases such as pulmonary hypertension5.
Pharmacoepigenomics, together with gene therapy, is something we must take into account, a new way for therapeutic strategies designing: may it be the next generation of treatments against cardiovascular diseases? We shall see!
References
- Wahlquist C., Agustin Rojas-Muñoz, Changwon Kho, Ahyoung Lee, Shinichi Mitsuyama, Alain van Mil, Woo Jin Park, Joost P. G. Sluijter, Pieter A. F. Doevendans & Roger J. Hajjar & (2014). Inhibition of miR-25 improves cardiac contractility in the failing heart, Nature, 508 (7497) 531-535. DOI: http://dx.doi.org/10.1038/nature13073 ↩
- High throughput measurement of Ca²⁺ dynamics for drug risk assessment in human stem cell-derived cardiomyocytes by kinetic image cytometry. Cerignoli F, Charlot D, Whittaker R, Ingermanson R, Gehalot P, Savchenko A, Gallacher DJ, Towart R, Price JH, McDonough PM, Mercola M. J Pharmacol Toxicol Methods. 2012 Nov-Dec;66(3):246-56. doi: 10.1016/j.vascn.2012.08.167. PMID: 22926323 ↩
- Engineering adeno-associated viruses for clinical gene therapy. Kotterman MA, Schaffer DV. Nat Rev Genet. 2014 Jul;15(7):445-51. doi: 10.1038/nrg3742. PMID: 24840552 ↩
- Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ; Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators. Circulation. 2011 Jul 19;124(3):304-13. doi: 10.1161/CIRCULATIONAHA.111.022889. PMID: 21709064 ↩
- Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Zhao L, Chen CN, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, Wilkins MR. Circulation. 2012 Jul 24;126(4):455-67. doi: 10.1161/CIRCULATIONAHA.112.103176. PMID: 22711276 ↩
1 comment
[…] aplikazioak askotarikoak dira. Batzuek aplikazio medikoak dira zuzenean. Eduardo Oliverrek Against a failing heart, pharmacoepigenomics artikuluan kardiopatien tratamenduetan farmakoepigenomikaren erabilerari buruz hitz egiten […]