How to regenerate a functional heart using 3D printing
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE
Cardiovascular diseases currently form the most important class of non-contagious diseases and a leading cause of mortality in industrialized nations 1. Specifically, coronary heart disease is the first cause of death among the cardiovascular diseases 2. The myocardial infarction (MI), commonly known as a “heart attack”, occurs when blood flow decreases or stops in a coronary artery of the heart (this artery is responsible to supply oxygen-rich blood and nutrients to the heart). This interruption of blood supply if it is not solved causes fatal damage to the heart muscle.
The current treatments for coronary occlusion are the surgical implant of “stents” and the administration of thrombolytic agents. A “stent” is a tiny and expandable metal net that it is placed into the affected area of the artery to help to prevent from narrowing or closing again. The thrombolytic agents are drugs that its mechanism of action relies on its capacity to dissolve the blood clots that may block a coronary artery and, then, cause the heart attack. Despite these treatments, insufficient blood flow occurs frequently 3. Unfortunately, to date, heart transplantation is the only strategy for patients with a very damaged heart or in an end-stage heart failure. Since the number of cardiac donors is very limited, there is an urgent need to develop new alternative approaches to regenerate the infarcted heart 4. At this point, the engineering of cardiac tissue provides an interesting approach in which a cardiac area can be transplanted onto the damaged heart, leaving a functional living area that help to regenerate the heart. However, one of the difficulties that arises from this technique is the generation, at the same time, of a cardiac tissue containing functional blood vessels essentials for the appropriate blood supply to the muscle area, breaking this vicious circle.
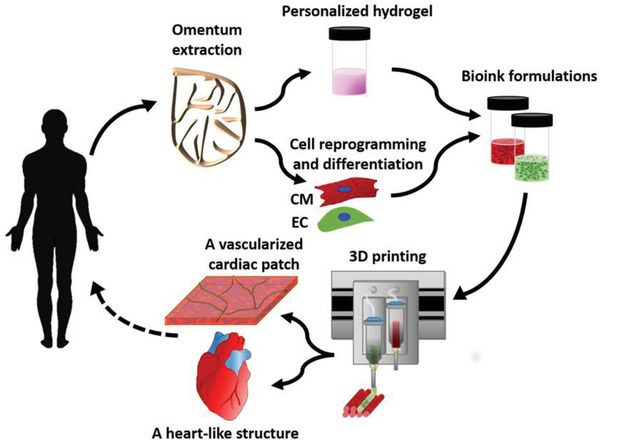
Recently, the group of Nadar Noor and collaborators have managed to solve the above-mentioned problem, finding a strategy to develop cardiac portions with major and functional blood vessels in one step using 3D printing 5. 3D printing is the construction of a three-dimensional object (an organ or a portion of an organ in our case) adding successive layers of different materials (alive cells in our case). The authors use a digital 3D model which is generated with specific mathematical models and a specialized software. To create vascularized cardiac portions, the authors used “omentum” tissue (a large, flat adipose tissue layer localized on the surface of the intra-peritoneal organs). Cells of the omentum are separated from the extracellular matrix (the region of tissue that serves as “scaffold” where the cells are attached). Once the matrix is decellularized, then is processed into a “personalized” hydrogel composed of collagenous fibers to create the morphological shape of the required portion of structure 6. Then, the previously recovered cells from the omentum are undifferentiated and converted into pluripotent stem cells (also known as “iPSCs”). These iPSCs are derived from differentiated cells and under specific cues and treatments are “reprogrammed” to gain “pluripotent” capabilities, that means to become able to generate different cell types of the organism. So, now the idea is to differentiate them to the main lineages of cells that make up the heart: the cardiac muscle cells called “cardiomyocytes” (CMs) and the endothelial cells (ECs) that will form the blood vessels. The main advantage of the use of iPSCs and cells derived from iPSCs is the absence of immune rejection, since both stem cells and the differentiated CMs and ECs are autologous (from the same patient).
Nadar Noor and collaborators mixed the personalized hydrogel with iPSC‐derived CMs to print the designed “engineered” cardiac tissue. Then, iPSC‐derived ECs were mixed with a 3D matrix called gelatin and a transient bioink to print the pattern of the designed vasculature. In bioprinting, a bioink is any natural or synthetic polymer selected for its biocompatible components. These characteristics temporarily or permanently support living cells to facilitate their adhesion, proliferation and differentiation process. Next, in order to fit a 3D cardiac portion for a specific patient, the portion of hearth, its dimensions and the blood vessel geometry were designed by computer‐aided design (CAD) software that used anatomical data from computed tomography (CT) images (CT is a diagnostic imaging test used to create detailed images of internal body parts). A 3D printer was used to print the parenchymal cell‐containing hydrogel simultaneously with gelatin that contains blood vessel‐forming cells to create a 3D, patient‐specific, vascularized area that had high cell viability. Upon incubation at 37 °C, the blood vessel‐forming cells adhered to the parenchymal cell‐containing hydrogel, while the gelatin became liquid dissolving and disappearing from the construct, leaving opened the blood vessels within the cardiac portions. Furthermore, liquid could be infused into the open vessels of the portions, indicating that their lumen structure was maintained. The function of the printed muscle cardiac area was demonstrated by the appropriate measure of calcium transients in the contracting engineered tissue.
Finally, to assess the presence and morphology of the printed cells in vivo, the engineered portions were transplanted to two layers of rat omentum. The cellularized areas were then extracted and histologically analyzed to study the microscopic anatomy of the portions. Then, parenchymal muscle cells were elongated, and aligned, with massive striation, which indicated their contractility potential. Furthermore, the printed blood vessels with open lumens (the lumen is the inside space of the blood vessels) were presented within the tissue, indicating their functionality.
Overall, taken together, these results demonstrate the “proof of concept” for the future use of the patient’s own cells and 3D printed materials in a fully personalized, contracting cardiac portions that closely fit to the patient’s biochemical and cellular properties, as well as for the anatomy of the patient. In conclusion, the authors pave the way for future bioengineering to recreate “spare parts” of clinically relevant cardiac portions for future therapies.
References
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M et al. Circulation. 2017; 135, e146. PMID: 28122885 DOI: 10.1161/CIR.0000000000000485 ↩
- European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J. 2018; 39: 508–579. PMID: 29190377. DOI: 10.1093/eurheartj/ehx628 ↩
- Why is the subendocardium more vulnerable to ischaemia? A new paradigm. Am J Physiol Heart Circ Physiol. 2011; 300: 1090-1100. PMID: 21169398. DOI: 10.1152/ajpheart.00473.2010 ↩
- Kittleson MM. Curr. New issues in heart transplantation for heart failure. Treat. Opt. Cardiovasc. Med. 2012; 14: 356-369. PMID: 22648352 DOI: 10.1007/s11936-012-0184-7 ↩
- Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. 2019; 6:1900344. PMID: 31179230. DOI: 10.1002/advs.201900344 ↩
- Edri R, Gal I. Noor N, Harel T, Fleischer S, Adadi N, Green O, Shabat D, Heller L, Shapira A. Personalized Hydrogels for Engineering Diverse Fully Autologous Tissue Implants. Adv. Mater. 2018; 31: 1803895. PMID: 30406960DOI: 10.1002/adma.201803895 ↩